Updates in Management and Timing of Dialysis in Acute Kidney Injury
Acute kidney injury (AKI) is a common complication in hospitalized patients and is associated with mortality, prolonged hospital length of stay, and increased healthcare costs. This paper reviews several areas of controversy in the identification and management of AKI. Serum creatinine and urine output are used to identify and stage AKI by severity. Although standardized definitions of AKI are used in research settings, these definitions do not account for individual patient factors or clinical context which are necessary components in the assessment of AKI. After treatment of reversible causes of AKI, patients with AKI should receive adequate volume resuscitation with crystalloid solutions. Balanced crystalloid solutions generally prevent severe hyperchloremia and could potentially reduce the risk of AKI, but additional studies are needed to demonstrate a clinical benefit. Intravenous albumin may be beneficial in patients with chronic liver disease either to prevent or attenuate the severity of AKI; otherwise, the use of albumin or other colloids (eg, hydroxyethyl starch) is not recommended. Diuretics should be used to treat volume overload, but they do not facilitate AKI recovery or reduce mortality. Nutrition consultation may be helpful to ensure that patients receive adequate, but not excessive, dietary protein intake, as the latter can lead to azotemia and electrolyte disturbances disproportionate to the patient’s kidney failure. The optimal timing of dialysis initiation in AKI remains controversial, with conflicting results from two randomized controlled trials.
© 2019 Society of Hospital Medicine
Acute kidney injury (AKI) is a common complication in hospitalized patients, affecting one in five inpatients1,2 and more than half of patients in intensive care units (ICU).3 The incidence of AKI appears to be increasing over time.4 Potential contributing factors include an aging population, rising prevalence of comorbid conditions such as heart failure and chronic kidney disease (CKD), using nephrotoxic agents, and increasing complexity of surgical procedures.5,6 AKI during a hospital stay is associated with a two to 10-fold increased risk of inhospital mortality,1,2,7-10 longer hospital length of stay,7,10 higher risk for hospital readmissions,11 and higher healthcare costs.7 Patients who survive an episode of AKI have a higher risk for CKD and dialysis-dependence,9 even after an episode of reversible AKI.12 Despite its clinical importance, several areas of controversy remain regarding the management of AKI and, in particular, the optimal timing of renal replacement therapy (RRT) in patients with AKI. The purpose of this manuscript is to review the approaches to diagnosis and management of AKI in hospitalized patients. We also review recent evidence regarding the timing of dialysis in patients with AKI. This journal recently reviewed the differential diagnosis and diagnostic evaluation of AKI, which is not covered here.13
DEFINITION OF ACUTE KIDNEY INJURY
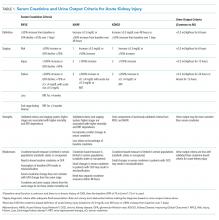
AKI refers to an acute change in kidney function characterized by an increase in serum creatinine and/or a reduction in urine output. It is a clinical syndrome caused by a broad range of etiologies and may be related to primary kidney pathology and/or systemic illness. Until 2004, there was no standard definition for AKI and over 30 different definitions were found in the literature, which resulted in wide variation in the reported incidence and outcomes of AKI and made it challenging to apply an evidence-based approach to patient care. In 2004, the Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE)14 criteria for AKI were proposed, which were modified to the Acute Kidney Injury Network (AKIN)15 criteria in 2007 (Table 1). Multiple studies show that the RIFLE and AKIN criteria for AKI are associated with higher mortality1,2,8,10 and increased risk for requiring RRT.1,10
International clinical practice guidelines for AKI were released by Kidney Disease: Improving Global Outcomes (KDIGO) in 2012, which included a standardized definition of AKI that was adapted from the previously validated RIFLE and AKIN definitions.16 Patients are considered to have AKI when the serum creatinine rises by as little as 0.3 mg/dL. It is notable that when the baseline serum creatinine is high, there is more inherent variability in the serum creatinine measurement; thus, patients with CKD have a higher risk of being misclassified as having AKI.17 Although the KDIGO definition for AKI is commonly used in research settings, components of this definition have not been well validated, and it is not widely used in clinical practice. Other renal professional societies still recommend an individualized approach to the diagnosis of AKI, taking into account other factors such as trajectories in kidney function, fluid balance, electrolyte abnormalities, comorbid conditions, and clinical context.18,19 While we endorse the KDIGO approach to the categorization of AKI severity, in practice, a more patient-centered approach is generally required to guide the optimal approach to determining the etiology of AKI and guiding management.