Things We Do for No Reason: Prescribing Docusate for Constipation in Hospitalized Adults
© 2019 Society of Hospital Medicine
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
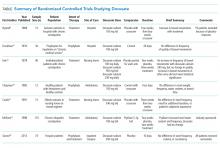
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24