Predictors of Long-Term Opioid Use After Opioid Initiation at Discharge From Medical and Surgical Hospitalizations
Opioid analgesics may be initiated following surgical and medical hospitalization or in ambulatory settings; rates of subsequent long-term opioid (LTO) use have not been directly compared. This retrospective cohort study of the Veterans Health Administration (VHA) included all patients receiving a new outpatient opioid prescription from a VHA provider in fiscal year 2011. If a new outpatient prescription was filled within 2 days following hospital discharge, the initiation was considered a discharge prescription. LTO use was defined as an episode of continuous opioid supply lasting a minimum of 90 days and beginning within 30 days of the initial prescription. We performed bivariate and multivariate analyses to identify the factors associated with LTO use following surgical and medical discharges. Following incident prescription, 5.3% of discharged surgical patients, 15.2% of discharged medical patients, and 19.3% of outpatient opioid initiators received opioids long term. Medical and surgical patients differed; surgical patients were more likely to receive shorter prescription durations. Predictors of LTO use were similar in medical and surgical patients; the most robust predictor in both groups was the number of days’ supply of the initial prescription (odds ratio [OR] = 1.24 and 95% confidence interval [CI], 1.12-1.37 for 8-14 days; OR = 1.56 and 95% CI, 1.39-1.76 for 15-29 days; and OR = 2.59 and 95% CI, 2.35-2.86 for >30 days) compared with the reference group receiving ≤7days. Rates of subsequent LTO use are higher among discharged medical patients than among surgical patients. Characteristics of opioid prescribing within the initial 30 days, including initial dose and days prescribed, were strongly associated with LTO use.
RESULTS
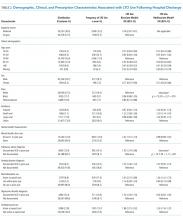
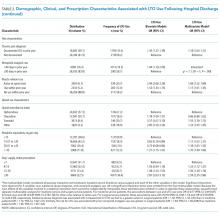
Days’ supply was associated with LTO use in a dose-dependent fashion relative to the reference category of ≤7 days: OR of 1.24 (95% CI, 1.12-1.37) for 8 to 14 days; OR of 1.56 (95% CI, 1.39-1.76) for 15 to 29 days; and OR of 2.59 (95% CI, 2.35-2.86) for 30 days (Table 2). LTO risk was higher among patients with an estimated dose of ≥15 morphine equivalents per day (MED) compared with those with doses of <15 equivalents (OR = 1.11; 95% CI, 1.02-1.21); patients who received >45 MED were at the greatest risk (OR = 1.70; 95% CI, 1.49-1.94).
DISCUSSION
The observation that subsequent LTO use occurs more frequently in discharged medical patients than surgical patients is consistent with the findings of Calcaterra et al.1 that among patients with no surgery versus surgery during hospitalization, opioid receipt at discharge resulted in a higher adjusted OR (7.24 for no surgery versus 3.40 for surgery) for chronic opioid use at 1 year. One explanation for this finding may be an artifact of cohort selection in the study design: patients with prior opioid use are excluded from the cohort, and prior use may be more common among surgical patients presenting for elective inpatient surgery for painful conditions. Previous work suggests that opioid use preoperatively is a robust predictor of postoperative use, and rates of LTO use are low among patients without preoperative opioid exposure.6
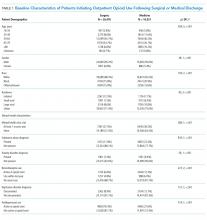
Demographic characteristics associated with persistent opioid receipt were similar to those previously reported.5,8,9 The inclusion of medication classes indicated in the treatment of mental health or pain conditions (ie, antidepressants, benzodiazepines, muscle relaxants, and nonopioid analgesics) resulted in diagnoses based on ICD-9 codes being no longer associated with LTO use. Severity or activity of illness, preferences regarding pharmacologic or nonpharmacologic treatment and undiagnosed or undocumented pain-comorbid conditions may all contribute to this finding. Future work studying opioid-related outcomes should include variables that reflect pharmacologic management of comorbid diagnoses in the cohort development or analytic design.
The strongest risk factors were potentially modifiable: days’ supply, dose, and concurrent medications. The measures of opioid quantity supplied are associated with subsequent ongoing use and are consistent with recent work based on prescription drug–monitoring data in a single state14 and in a nationally representative sample.15 That this relationship persists following hospital discharge, a scenario in which LTO use is unlikely to be initiated by a provider (who would be expected to subsequently titrate or monitor therapy), further supports the potential to curtail unintended LTO use through judicious early prescribing decisions.
We assessed only opioids that were supplied through a VA pharmacy, which may lead to the misclassification of patients as opioid naive for inclusion and an underestimation of the rate of opioid use following discharge. It is possible that differences in the rates of non-VA pharmacy use differ in medical and surgical populations in a nonrandom way. This study was performed in a large, integrated health system and may not be generalizable outside the VA system, where more discontinuities between hospital and ambulatory care may exist.