How Does Your PICCOMPARE? A Pilot Randomized Controlled Trial Comparing Various PICC Materials in Pediatrics
BACKGROUND: Despite the popularity of peripherally inserted central catheters (PICCs), recent literature highlights their potential injurious complications. Innovative PICC materials have been developed to prevent thrombosis and infection formation (Endexo®) and antireflux valves to prevent occlusion (pressure-activated safety valve®). No large randomized controlled trial has assessed these technologies. Our primary aim was to evaluate the feasibility of a large randomized controlled efficacy trial of PICC materials and design to reduce PICC complication in pediatrics.
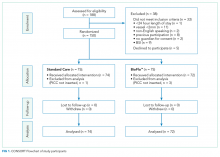
METHODS: A randomized controlled feasibility trial was undertaken at the Lady Cilento Children’s Hospital in South Brisbane, Australia, between March 2016 and November 2016. Consecutive recruitment of 150 pediatric participants were randomly assigned to receive either (1) polyurethane PICC with a clamp or (2) BioFlo® PICC (AngioDynamics Inc, Queensbury, NY). Primary outcomes were trial feasibility, including PICC failure (thrombosis, occlusion, infection, breakage, or dislodgement). Secondary outcomes were PICC complications during use.
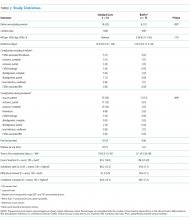
RESULTS: Protocol feasibility was established, including staff and patient acceptability, timely recruitment, no missing primary outcome data, and 0% attrition. PICC failure was 22% (16 of 74, standard care) and 11% (8 of 72, BioFlo®) corresponding to 12.6 and 7.3 failures per 1000 hours (risk ratio 0.58; 95% confidence interval, 0.21-1.43; P = .172). PICC failures were primarily due to thrombosis (standard care 7% versus BioFlo® 3%) and complete occlusion (standard care 7% versus BioFlo® 1%). No blood stream infections occurred. Significantly fewer patients with BioFlo® had PICC complications during use (15% vs 34%; P = .009).
CONCLUSION: BioFlo® PICCs appear potentially safer for pediatrics than traditional standard care PICCs with a clamp. Further research is required to definitively identify clinical, cost-effective methods to prevent PICC failure and improve reliability.
© 2018 Society of Hospital Medicine
Ethics
The Children’s Health Service District, Queensland (Human Research Ethics Committee/15/QRCH/164), and Griffith University (2016/077) Human Research Ethics Committees provided ethics and governance approval. Informed consent was obtained from parents or legal guardians, with children providing youth assent if they were 7 years or older, dependent upon cognitive ability.
RESULTS
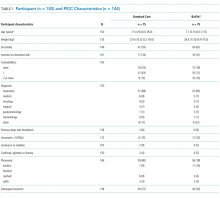
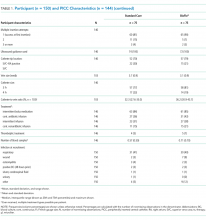
Participant and PICC Characteristics
Feasibility Outcomes
PICC Failure and Complications
As per supplementary Table 1, univariate Cox regression identified PICC failure as significantly associated with tip placement in the proximal superior vena cava (SVC) compared to the SVC–right atrium junction (HR 2.61; 95% CI, 1.17-5.82; P = .024). Reduced risk of PICC failure was significantly associated with any infusion during the dwell (continuous fluid infusion, P = .007; continuous antibiotic, P = .042; or intermittent infusion, P = .046) compared to no infusion. Other variables potentially influencing the risk of failure included PICC insertion by nurse specialist compared to consultant anesthetist (HR 2.61; 95% CI, 0.85-5.44) or registrar (HR 1.97; 95% CI, 0.57-6.77). These differences were not statistically significant; however, baseline imbalance between study groups for this variable and the feasibility design preclude absolute conclusions.
DISCUSSION
This is the first pilot feasibility trial of new PICC materials and valve design incorporated in the BioFlo® PICC in the pediatric population. The trial incorporated best practice for randomized trials, including using a concurrent control group, centralized and concealed randomization, predetermined feasibility criteria, and a registered and published trial protocol.17 As in other studies,15,24,34 PICC failure and complication prevalence was unacceptably high for this essential device. Standard care PICCs failed twice as often as the new BioFlo® PICCs (22% vs 11%), which is a clinically important difference. As researchers in a pilot study, we did not expect to detect statistically significant differences; however, we found that overall complications during the dwell occurred significantly more with the standard care than BioFlo® PICCs (P = .009).