Urothelial Carcinoma: Muscle-Invasive and Metastatic Disease
First-Line Management: Metastatic Disease
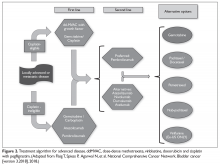
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.