HER2-Positive Breast Cancer: Current Management
Introduction
Breast cancer is the second leading cause of cancer deaths among women in the United States, according to the SEER database. It is estimated that 1 in 8 women will be diagnosed with breast cancer at some point during their lifetime (12.4% lifetime risk).1,2 Because breast tumors are clinically and histopathologically heterogeneous, different diagnostic and therapeutic approaches are required for each subtype. Among the subtypes, tumors that are positive for human epidermal growth factor receptor 2 (HER2) account for approximately 15% to 20% of all newly diagnosed localized and metastatic invasive breast tumors.3,4 Historically, this subset of tumors has been considered the most aggressive due to a higher propensity to relapse and metastasize, translating into poorer prognosis compared with other subtypes.5–7 However, with the advent of HER2-targeted therapy in the late 1990s, prognosis has significantly improved for both early- and late-stage HER2-positive tumors.8
Pathogenesis
The HER2 proto-oncogene belongs to a family of human epidermal growth factor receptors that includes 4 transmembrane tyrosine kinase receptors: HER1 (also commonly known as epidermal growth factor receptor, EGFR), HER2, HER3, and HER4. Another commonly used nomenclature for this family of receptors is ERBB1 to ERBB4. Each of the receptors has a similar structure consisting of a growth factor–binding extracellular domain, a single transmembrane segment, an intracellular protein-tyrosine kinase catalytic domain, and a tyrosine-containing cytoplasmic tail. Activation of the extracellular domain leads to conformational changes that initiate a cascade of reactions resulting in protein kinase activation. ERBB tyrosine receptor kinases subsequently activate several intracellular pathways that are critical for cellular function and survival, including the PI3K-AKT, RAS-MAPK, and mTOR pathways. Hyperactivation or overexpression of these receptors leads to uncontrolled cell growth and proliferation, and eventually cancerogenesis.9,10
HER2 gene amplification can cause activation of the receptor’s extramembranous domain by way of either dimerization of two HER2 receptors or heterodimerization with other ERBB family receptors, leading to ligand-independent activation of cell signaling (ie, activation in the absence of external growth factors). Besides breast cancer, HER2 protein is overexpressed in several other tumor types, including esophageal and gastric adenocarcinomas, colon and gynecological malignancies, and to a lesser extent in other malignancies.
Biomarker Testing
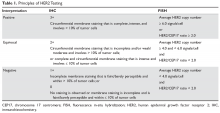
All patients with newly diagnosed breast cancer should have their tumor tissue submitted for biomarker testing for estrogen receptors (ER), progesterone receptors (PR), and HER2 overexpression, as the result this testing dictates therapy choices. The purpose of HER2 testing is to investigate whether the HER2 gene, located on chromosome 17, is overexpressed or amplified. HER2 status provides the basis for treatment selection, which impacts long-term outcome measures such as recurrence and survival. Routine testing of carcinoma in situ for HER2 expression/amplification is not recommended and has no implication on choice of therapy at this time.
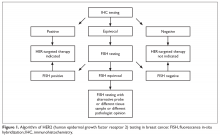
In 2013, the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) updated their clinical guideline recommendations for HER2 testing in breast cancer to improve its accuracy and its utility as a predictive marker.11 There are currently 2 approved modalities for HER2 testing: detection of HER2 protein overexpression by i
Fluorescence in-situ hybridization (FISH) testing assesses for HER2 amplification by determining the number of HER2 signals and