Locally Advanced Pancreatic Cancer
CANCER BIOLOGY AND PATHOLOGY
The pathologic predecessor of PDA is pancreatic intraepithelial neoplasia (PIN). With further dysplastic changes that result from increasing genetic alterations, these precancerous lesions progress from low- to high-grade and finally to adenocarcinoma. More than 90% of all PINs across all grades have oncogenic KRAS mutations.28 Additionally, inactivating mutations in the tumor suppressor genes SMAD4, p53, and CDKN2A are found with increasing frequency in higher grade PINs. The frequency and presence of mutations in both oncogenes and tumor suppressor genes in precursor neoplasias mirror the genetic mutations noted in advanced PDA.29 Among all mutations, KRAS is the most common and most functionally important for pancreatic cancer cell survival. KRAS mutations not only have profound effects on downstream mediators of tumor growth and metastasis, but they are implicated in reprograming of cellular metabolism.30,31
Pancreatic adenocarcinoma has a unique microenvironment that makes it a difficult target for current therapeutic modalities. First, it is one of the most stroma-rich malignancies. The dense stroma surrounding pancreatic tumor cells leads to increased tumor pressures and alterations in tumor vascular perfusion.32 It also serves as a barrier that prevents chemotherapeutic drugs from reaching the tumor cells. Thus, clinical trials are under way to investigate agents such has hyaluronidase, which may degrade components of the extracellular matrix that supports thestromal environment. Additionally, there is data to suggest that the microenvironment of PDA downregulates immune monitoring, leading to further tumor growth.27,33 The molecular, cellular, and immunologic complexity of PDA may contribute to its resistance to traditional therapeutics.
EVALUATION AND DIAGNOSIS
CASE PRESENTATION
A 61-year-old man with a history of type 2 diabetes mellitus and chronic tobacco use presents to the emergency department (ED) with a 4-month history of progressively worsening abdominal discomfort and fatigue. He has also noticed darkening of his urine and slight yellow discoloration of his eyes. His weight measured 5 months ago in his primary care physician’s office was 91 kg (200 lb, BMI 29.5) and in the ED is 75 kg (165 lb, BMI 24.4). He has noticed bulky, malodorous, oily stools for about 2 months. Preliminary laboratory studies reveal elevated levels of total bilirubin (2.7 mg/dL) and alkaline phosphatase (204 IU/L). Transabdominal ultrasound (US) is obtained and reveals a 3-cm pancreatic mass with biliary tract dilation.
,Does this patient have pancreatic cancer?
CLINICAL SIGNS AND SYMPTOMS
Establishing the diagnosis of pancreatic cancer in a patient who presents with a high index of suspicion is critical. Patients with pancreatic cancer usually present after a period of nonspecific and vague symptoms, which typically are experienced as abdominal discomfort, weight loss, and weakness. It is estimated that approximately 25% of patients may complain of vague abdominal pain up to 6 months prior to diagnosis. Up to 15% of patients may seek medical attention more than 6 months prior to establishing a diagnosis of PDA.34 The most common symptoms associated with pancreatic cancer in order of decreasing reported frequency are weight loss, anorexia, abdominal/epigastric pain, dark-colored urine, jaundice, nausea, back pain, and diarrhea with associated steatorrhea.35 Upwards of 15% of patients present with painless jaundice, a term that is often associated with pancreatic cancer.36 On exam these patients may have scleral icterus, sublingual jaundice, epigastric pain on palpation, weight loss, hepatomegaly, lymphadenopathy and a nontender, distended, palpable gallbladder (also known as Courvoisier sign).34 Abdominal signs and symptoms arise from tumor growth into surrounding vessels, tissues, and ducts within the abdominal cavity. Compression of the common bile duct accounts for the development of jaundice. Tumor growth around the stomach and duodenum can lead to delayed gastric emptying and subsequently nausea and vomiting. Constriction of the pancreatic duct leads to pancreatic insufficiency, precipitation of weight loss, and steatorrhea. Pancreatic insufficiency can worsen abdominal pain, and lead to increased weight loss and flatulence.
Less common symptoms include pain, erythema, and edema involving the lower extremities, which may be reflective of migratory thrombophlebitis (commonly known as Trousseau syndrome). Thromboembolic disease, including pulmonary embolism, portal vein, and deep vein thromboses are frequently encountered complications of pancreatic cancer. The incidence of thromboembolic events in patients with PDA has been reported to be as high as 54%.37 Of all signs encountered, weight loss is the most common and most profound. Patients with advanced PDA have severe degrees of cachexia. Some patients present with as much as a 5 kg/m2 decrease in their BMI from pre-illness baseline BMI, and lose another 3 to 4 kg/m2 through disease progression.38 At the time of diagnosis, many patients have already undergone significant weight loss, which can have substantial implications on treatment planning and clinical outcomes.
What other studies can be done to assist in making the diagnosis?
LABORATORY ABNORMALITIES AND TUMOR MARKERS
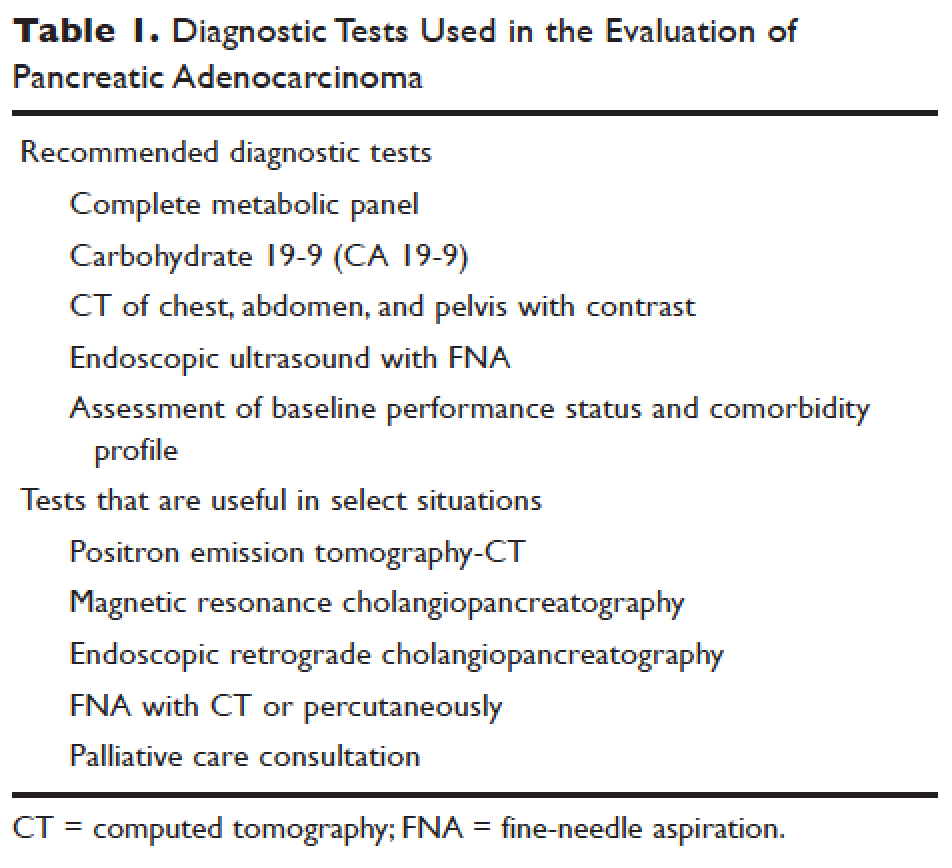
Elevations in alkaline phosphatase, γ-glutamyltransferase (GGT), serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), and direct fractions of bilirubin are common in patients with PDA. Patients will usually have an obstructive pattern on their liver panel, with predominant elevations in direct bilirubin, alkaline phosphatase, and GGT, as compared with AST and ALT. Other baseline laboratory studies, including a complete blood count and basic metabolic panel, should be obtained because patients commonly have thrombocytosis, anemia, and electrolyte abnormalities due to the tumor itself and pancreatic insufficiency (Table 1).