Development, implementation, and evaluation of a prostate cancer supportive care program
Many men who are diagnosed with prostate cancer face long-term treatment-related health effects that will affect their quality of life and have cost implications for the health system. In this article, we describe and assess the use of and satisfaction with the Prostate Cancer Supportive Care (PCSC) Program, which is a comprehensive, evidence-based, modular program that aims to address these concerns. We include data from patient medical records, PCSC Program registration forms and attendance records, and anonymized participant feedback forms. We examine the clinical and sociodemographic characteristics of program participants, program participation rates, and satisfaction with individual program modules. Among the 1,269 registrants, 1,206 (84%) participated in the program. Modules that provided information on prostate cancer and treatment options and offered sexual health support had the most participants (29% and 55% of total program participants, respectively). Satisfaction with all program components was high among both survivors and their partners (average score, 3.6 out of 4). Robust evaluations of the program's effects on quality of life and health system costs are ongoing. There is a growing need to provide consistent and comprehensive support to prostate cancer survivors and their partners and families. As such, we recommend that alongside direct oncologic care, clinicians assess their patients' needs for supportive care services and refer them to programs that will provide comprehensive support throughout the disease and treatment journey. Funding The Michael Smith Foundation for Health Research (grant number 16605) and Prostate Cancer Canada (grant number PDF2016-1270)
Accepted for publication November 20, 2018
Correspondence Lindsay Hedden, PhD; lindsay.hedden@ubc.ca
Disclosures Dr Elliot served on the board for Aceras, and has given a lecture for Pentopharm. Dr Higano has received research grants from Aptero, Aragon, Astellas, Astra-Zenica, Bayer, Dentreon, Hoffman-Laroche, Medivation, and Pfizer. Dr Higano also served on the advisory board or in a consultancy role for Astellas, Bayer, Blue Earth, Cloris, Orion, and Tolmar.
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0438
Methods
Data
We obtained sociodemographic, diagnostic, and treatment information as well as clinic visit records for all PCSC Program registrants from the electronic medical record held at the VPC. Clinical variables included age at diagnosis, Gleason score, and primary treatment modality (including active surveillance and ADT use). The Gleason score determines the aggressiveness of a patient’s prostate cancer based on biopsy results. A score of 6 or less indicates that the disease is likely to grow slowly. A grade of 7 is considered intermediate risk (with primary score of 3 and secondary 4 being lower risk than those with a primary score of 4 and secondary of 3). A Gleason score of 8 or higher indicates aggressive disease that is poorly differentiated or high grade. Sociodemographic characteristics included age, travel distance to the clinic, and income quintile. We obtained attendance records for the modular education sessions from the program’s database. Patients who did not have any medical visits at the VPC (referred to henceforth as non-VPC patients) did not have a clinic record, so we excluded them from the subset of the analyses that depended on specific clinical variables.
All patients and partners who participate in any PCSC Program education sessions (introduction to prostate cancer, sexual health, nutrition, exercise, ADT, and pelvic-floor physical therapy) are asked to complete voluntary, anonymous feedback forms. These forms assess participant satisfaction using a series of Likert-based and Boolean response items as well as qualitative commentary. They include questions that assess the timing, structure, and content of each session.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statistical approach
Descriptive statistics were used to analyze participant characteristics, program participation rates, and participant satisfaction. For each module’s education session, we compared the overall satisfaction between patients and partners using t tests. We also compared the level of satisfaction across the different modules using a 1-way analysis of variance. For the sexual health and pelvic-floor physical therapy sessions, we compared satisfaction between participants who attended the education sessions before to those who attended following their primary treatment using t tests. We provide the eta squared (for analyses of variance) and Cohen d (for t tests) to provide an effect size estimate of any significant differences observed.
Results
Participants
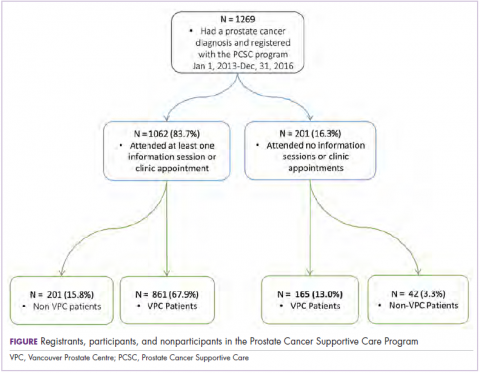
From the program’s founding in January of 2013 to December 31, 2016, a total of 1,269 patients registered (an average of 317 patients a year). Of those, 1,026 (80.9%) had at least 1 prostate cancer–related visit at the VPC. The remaining 243 (19.1%) were non-VPC patients (Figure). Overall, 1,062 men (83.4%) who registered with the program went on to attend at least 1 education session or clinic appointment.
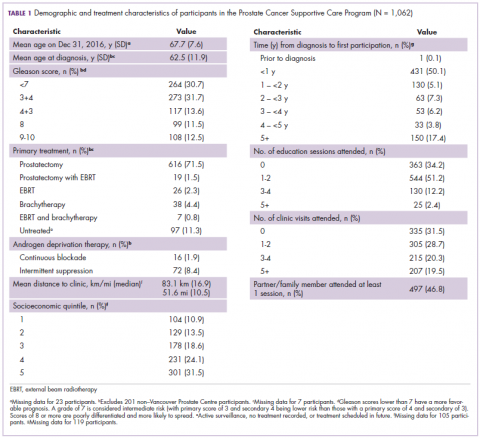
Average age among male program participants was 67.7 years, and age at diagnosis was 62.5 years (Table 1). In all, 273 men (31.7%) had Gleason 3+4, and 117 (13.7%) had Gleason 4+3. Most of the participants (76.9%) elected to undergo radical prostatectomy for primary treatment. Ninety-five men (8.9%) received at least some ADT treatment as an adjunct to radiation or to treat recurrent disease. Participants traveled an average of 83.1 km (51.6 miles; median, 6.9 km and 10.5 miles, respectively) to attend the program; 10% of participants traveled further than 112 km (70 mi) to the clinic. One hundred and four (10.9%) and 301 (31.5%) of registrants were in the lowest and highest income quintiles respectively. Four hundred and ninety-seven (46.8%) attended at lesson 1 session or clinic appointment with a partner or family member.