Effect of time of admission to treatment initiation on outcomes of patients with acute myeloid leukemia: a tertiary care referral center experience
Background The time from diagnosis of acute myeloid leukemia (AML) to initiation of treatment could affect patient outcomes, but findings from previous studies have been mixed.
Objective To analyze the impact of the time from adm ission to treatment initiation (TAT) on overall survival (OS) and event-free survival (EFS) in patients who are newly diagnosed with AML.
Methods A retrospective review of the records of all newly diagnosed AML patients treated at the Oklahoma University Health Sciences Center from January 2000 through June 2015 was conducted. Inclusion criteria also included age ≥18 years and available insurance data. Data on patient characteristics, laboratory values, pathology, treatment, response, and survival were obtained from the electronic medical records.
Results In all, 154 patients were divided into 2 groups: those with a TAT of 0-4 days (n = 109) and those with a TAT of >4 days (n = 45). The median OS of the TAT 0-4 days group and the TAT >4 days group was 1.3 years and 0.57 years, respectively(P = .0207), and the median EFS for the groups was 1.21 years and 0.57 years, respectively (P = .0392). That association remained significant in a multivariate analysis adjusting for age, white blood cell count, molecular risk group, and undergoing allogeneic stem cell transplant.
Limitations Study limitations include a small sample size and a short median follow-up time.
Conclusion Patients with AML who are treated more than 4 days after admission have a lower OS and EFS compared with patients treated within 0-4 days of admission.
Funding/sponsorship None
Accepted for publication September 13, 2018
Correspondence Sami Ibrahimi, MD; Sami-Ibrahimi@ouhsc.edu
Disclosures Cherry is on the advisory board of Gilead. The remaining authors report no disclosures or conflicts of interest.
Citation JCSO 2018;16(5):e188-e193
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0428
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
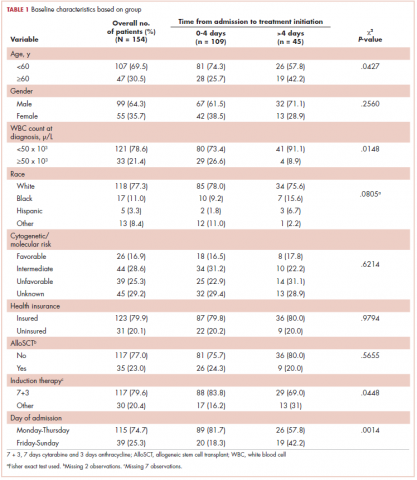
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).