Striking rash in a patient with lung cancer on a checkpoint inhibitor
Accepted for publication February 14, 2018
Correspondence Reinhold Munker, MD; rmunker@tulane.edu or
Georges E Tanios; MDgtanios@tulane.edu
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2018;16(3):e159-e162
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0403
Submit a paper here
Lung cancer remains the most common cause of cancer death in the United States and worldwide.1 Despite advances in the treatment of the disease and development of targeted therapy, the 5-year overall survival in stage IV non–small-cell lung cancer remains poor, ranging from 6% to 10%.2 More recently, checkpoint inhibitors have had a major impact on the treatment of lung cancer. Nivolumab was the first program cell death protein-1 (PD-1) inhibitor approved for malignant melanoma.3 In July 2015, it was approved as a second-line treatment of squamous cell carcinoma of the lung.4 Since then, the use of nivolumab has extended to other malignancies such as head and neck cancer, renal cell carcinoma, and the list continues to expand. In lung cancer, it demonstrated superior overall survival of 9 months, compared with 6 months with docetaxel.4 Other checkpoint inhibitors such as pembrolizumab5 and atezolizumab6 were subsequently developed, and are also used in the treatment of lung cancer.
Serious potential autoimmune complications arise in up to 30% of patients treated with PD-1 inhibitors. Dermatologic toxicity is the most common immune-related adverse event in these patients. In addition to vitiligo, most common is a reticular maculopapular rash on the trunk and extremities. Other adverse events, such as photosensitivity, alopecia, xerosis, and hair color changes, are reported less frequently.7 We report here a case of rash at an unusual location (auricular and periauricular) with skin exfoliation mimicking other common skin conditions such as eczema and psoriasis.
Case presentation and summary
A 57-year-old woman with a history of cerebrovascular accident with residual left lower-leg paresis presented for acute onset expressive aphasia in the absence of other constitutional or neurological findings. Magnetic resonance imaging of the brain showed a posterior, left parietal lobe lesion of 1.6 cm with intralesional hemorrhage and surrounding edema suggestive of brain metastasis. The patient had a 35 pack-year history of smoking. A staging work-up with computed-tomographic (CT) scans showed a spiculated enhancing nodule in the superior segment of the right lower lobe plus mediastinal adenopathy.
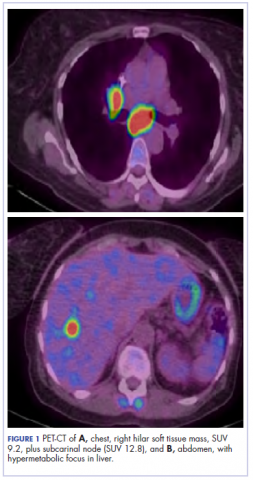
,The patient underwent a CT-guided core biopsy of the spiculated nodule, which was found to be consistent with adenocarcinoma of the lung. It was negative for EGFR mutation or ALK rearrangement. She received stereotactic radiosurgery to the left posterior parietal lesion, and after completion of radiation, was started on systemic chemotherapy with cisplatin plus pemetrexed for adenocarcinoma of the lung. She received 4 cycles of chemotherapy. Repeat imaging with a PET-CT showed interval increase of the mediastinal hypermetabolic lymphadenopathy with new hypermetabolic pretracheal lymph nodes and interval development of multiple liver metastases in the right and left lobes of the liver (Figure 1). She was started on second-line therapy with nivolumab at a dose of 240 mg every 2 weeks. The treatment was complicated initially by new onset grade 2 papular pruritic rash after cycle 2 of therapy. The rash involved the upper and lower extremities, sparing the palms, soles, trunk, abdomen, and the back. It resolved with treatment delay and topical steroids.
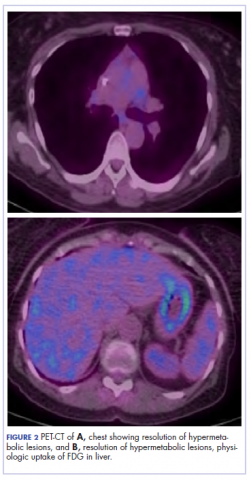
The patient resumed treatment with nivolumab after complete resolution of the rash. However, she developed grade 2 nephritis after cycle 5 with a creatinine level of 1.98 mg/dL (reference range, 0.6-1.2 mg/ dL). This was resolved after treatment with oral prednisone, at a starting dose of 1 mg/kg and tapered over 4 weeks. PET CT scans obtained after cycles 5 and 11 showed no metabolic activity in the mediastinum or the liver and markedly decreased uptake in the right lower lobe nodule, down to an SUV of 1.7 with no new nodules. An MRI of the brain was stable (Figure 2).
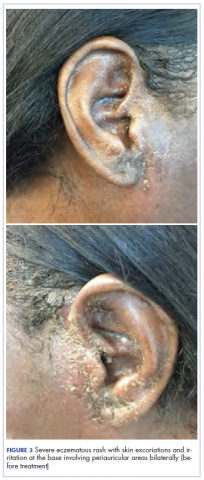
After cycle 16 of nivolumab, the patient developed a severe eczematous rash with excoriations at the base of both ears involving the periauricular and auricular areas bilaterally (Figure 3).
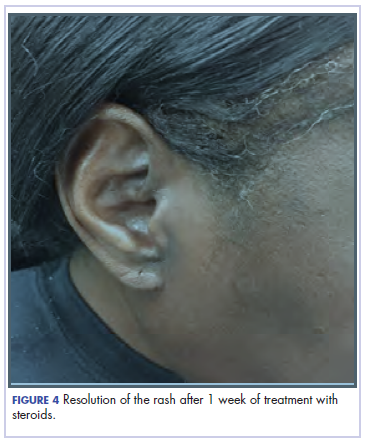
She completed 4 weeks of steroid therapy on a tapering schedule. Treatment with nivolumab was resumed afterward with no adverse autoimmune complications. At her last visit (25 months after initiating a PD-1 inhibitor), there was no clinical or radiologic evidence of lung cancer nor any of autoimmune adverse effects.