New myeloma drugs improve response and extend survival
In this interview, Dr David Henry, the Editor-in-Chief of The Journal of Community and Supportive Oncology, and Dr Ken Anderson, the Kraft Family Professor of Medicine at Harvard Medical School and an international thought leader and investigator in myeloma, discuss three cases of patients with myeloma that are indicative of the remarkable therapeutic advances in oncology in general, and in myeloma in particular. In the last 10-15 years, numerous approvals by the US Food and Drug Administration have transformed the treatment landscape for multiple myeloma by providing patients and oncologists with many new options and combination possibilities for treating the disease. And since many of the agents have been tested in advanced myeloma, their use has edged the disease toward initial management. The encouraging news is that in the new classes of drugs, and especially the second-generation drugs, response rates, progression-free disease, and overall survival are significantly better, with some combinations yielding response rates of up to 70%-80%, and overall and progression-free survival of up to 10 years.
Listen to the interview here
Received for publication November 2017
Published online first December 8, 2017
Correspondence David.Henry@uphs.upenn.edu
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2018;16(1):e53-e58
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0376
Related article
Immunotherapies shape the treatment landscape for hematologic malignancies
Submit a paper here
DR HENRY I thought we might discuss some cases of patients with myeloma, starting with a relatively simple case and ending with one that is a little more complicated. For the first case, we have a 56-year-old healthy man with IgG kappa myeloma whose work-up shows he has multiple lytic bone lesions. He has normal renal function, normal calcium, and he’s transplant-eligible by other health issues. I’ll leave the cytogenetics up to you if that changes your approach. How would you develop or pose some options for this man’s treatment to begin with?
DR ANDERSON It’s important to start out by saying that we, in myeloma, have many new classes of drugs and many new opportunities to choose from to treat this patient.1 As you know, we have proteasome inhibitors, the first-generation bortezomib, then carfilzomib and ixazomib. We have the immunomodulatory drugs (IMiDs), thalidomide, and now lenalidomide and pomalidomide. We have a histone deacetylase (HDAC) inhibitor approved called panobinostat, and we have 2 monoclonal antibodies approved, elotuzumab and daratumumab. These classes of medicine have made it possible for 20 different Food and Drug Administration (FDA) approvals in the last 10-15 years. These agents, having been tested in advanced myeloma, have moved toward initial management.
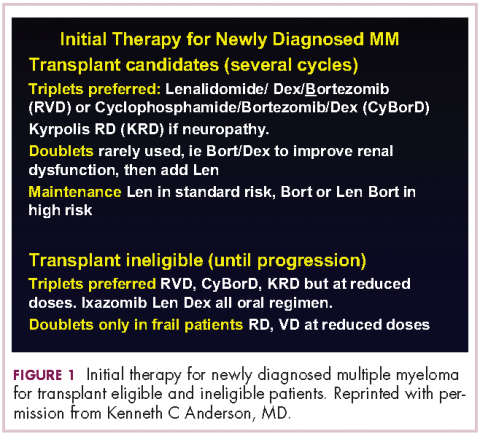
This person is 50 years old. He has adequate liver, heart, lung, and kidney function, so he would be eligible for high-dose therapy and stem-cell transplantation. In terms of initial management, there are many options (Figure 1). We strongly recommend that triplet therapy be used initially. The most common triplets would be lenalidomide, bortezomib, and dexamethasone (RVD)2,3 or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).4 If this man had neuropathy, perhaps carfilzomib, the second-generation proteasome inhibitor, with lenalidomide and dexamethasone could have been used. Why do we use these? The extent and frequency of response with these triplets is nearly universal overall response rate, with three-quarters very good partial and half-complete responses, including minimal residual disease negative responses. In this patient, we would therefore recommend treatment with either RVD or CyBorD for several cycles to maximal response.
,He would then have autologous stem cells collected, and it is still the standard of care to proceed to high-dose melphalan and a single high-dose therapy and stem-cell transplantation. The cytogenetics are important: if this patient has standard-risk multiple myeloma, then lenalidomide maintenance would be given after transplant. It is now FDA-approved for this purpose because it can prolong both progression-free and – most importantly – overall survival.5 Standard-risk cytogenetics might, for example, include hyperdiploidy or translocation 11;14. On the other hand, if his myeloma were high-risk and characterized, for example, by 17p deletion, we would carry out the same induction and transplantation, but we would alter the maintenance to incorporate a proteasome inhibitor. Lenalidomide and bortezomib, for example, could be combined. Early data show that using combined maintenance therapy with lenalidomide and bortezomib, can overcome the early relapses that are characteristic of high-risk disease.6
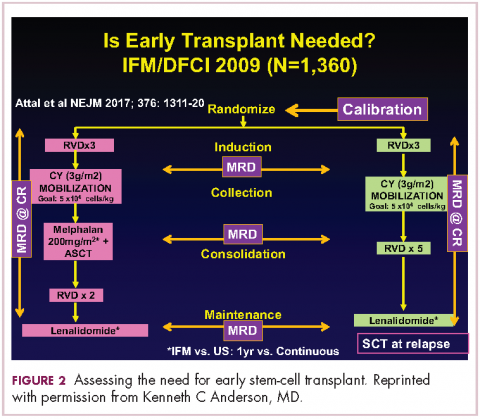
Because of the extent and frequency of response to combination novel therapies, we have undertaken with our French colleagues a clinical trial of RVD in newly diagnosed patients – such as this patient – followed by stem-cell collection in all patients (Figure 2). Then there is a randomization to either early high-dose therapy, melphalan, and autologous stem-cell transplantation, followed by lenalidomide maintenance; or in the other cohort, harvesting of stem cells, additional RVD, and then maintenance with lenalidomide, saving the stem-cell transplant for later.
The French portion of this trial was reported in the New England Journal of Medicine earlier in 2017.7 It showed that patients who received RVD, high-dose melphalan, stem-cell transplant, and had 1 year of lenalidomide maintenance, had a progression-free survival advantage of about 1 year, without an overall survival advantage; compared with those patients who received RVD and lenalidomide maintenance, saving the transplant for later. I would hasten to add that lenalidomide maintenance was given for only 1 year in this trial, and patients in the RVD-only or RVD-and-transplant arms of this trial relapsed after the lenalidomide maintenance was discontinued.
The American portion of this trial is identical. That is, RVD induction is being given and all patients have a stem-cell collection. Half of the patients then go to high-dose melphalan and stem-cell transplant early, and half of them have the transplant only later at the time of relapse. A major difference, however, is that in both the RVD-only and RVD-and-transplant cohorts, patients receive lenalidomide maintenance until progression. This trial has been ongoing since 2009 and is still ongoing, which tells us that patients in both arms – the RVD-only as well as the RVD-and-transplant arms – are doing well.
In the recent STAMINA trial, all patients underwent a single high-dose therapy and transplant. Then there was a randomization to lenalidomide maintenance only in 1 cohort; a randomization to consolidation with RVD posttransplant followed by lenalidomide maintenance in the second cohort; or a randomization to a second high-dose melphalan and stem-cell transplant followed by lenalidomide maintenance in the third cohort.8 I mention this because the outcomes in all 3 cohorts was similar.
I believe this tells us strongly that high-dose therapy and stem-cell transplantation twice – so-called tandem transplant – is no longer a major option in multiple myeloma. For now, however, in this patient, the standard of care would be to undergo induction therapy with triplet, novel combination treatment. Then, stem cells would be collected and high-dose therapy stem-cell transplant would be done, followed either by lenalidomide maintenance for standard disease or lenalidomide and bortezomib maintenance for high-risk disease. We won’t really know if we can delay transplant until the trials I’ve mentioned totally read out. In my clinical practice, if patients have had a major response to their induction therapy and have stem cells harvested, we can then offer them the opportunity to use maintenance therapy and save the transplant as a potential option for later, when myeloma relapses.