Concurrent ipilimumab and CMV colitis refractory to oral steroids
Accepted for publication September 5, 2017
Correspondence perrya@wustl.edu
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2018;16(1):e30–e33
©2018 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0368
Related articles
Effectiveness and safety of ipilimumab therapy in advanced melanoma...
Delayed response in ipilimumab therapy
Submit a paper here
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
,The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
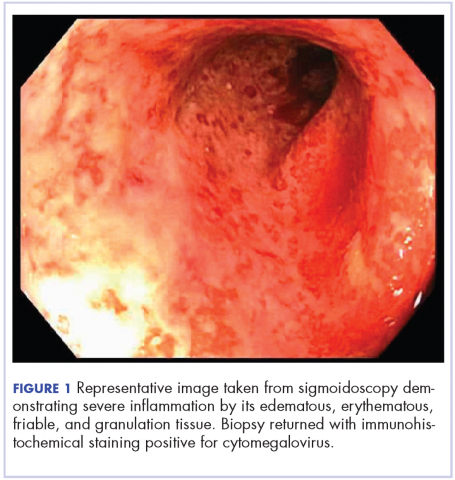
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
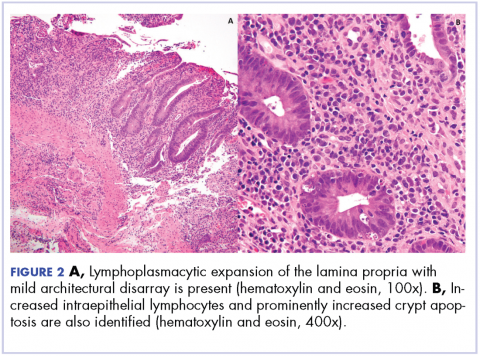
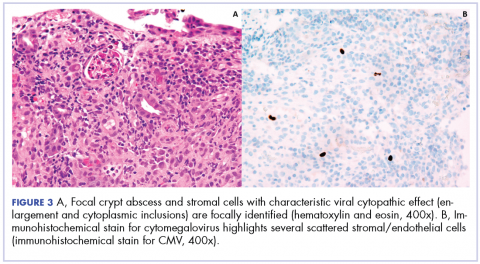
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.