Physician attitudes and prevalence of molecular testing in lung cancer
Background EGFR mutations and EML4-ALK rearrangements are key therapeutic targets in nonsquamous non–small-cell lung carcinoma (nsNSCLC). Current guidelines recommend testing all patients with advanced nsNSCLC (stages IIIB and IV).
Objective To evaluate physician attitudes about molecular testing for nsNSCLC and to determine the rate of testing, the effect of biopsy sample size, and prevalence of driver mutations.
Materials and methods In this retrospective study, 206 cases of advanced nsNSCLC were identified from the tumor registry from 3 hospitals within a health network (February 2011-February 2013). EGFR and ALK testing was performed using commercial laboratories and mutation prevalence was determined. A survey was sent to practitioners who care for patients with lung cancer to evaluate their attitudes toward molecular testing.
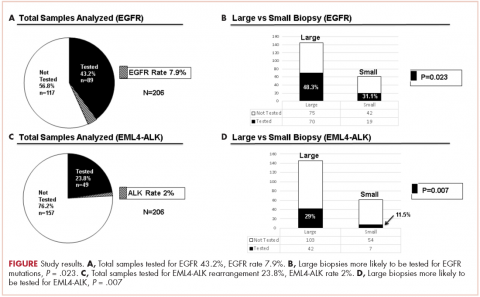
Results The prevalence of EGFR mutation (7.8%) and ALK rearrangement (2%) was lower than reported in the literature. Large biopsy samples were more likely to be analyzed for EGFR mutations and ALK rearrangements (P = .023 and P = .007, respectively) than were smaller samples. There was a high level of agreement among survey respondents that mutation testing was essential. Nevertheless, we found that fewer than half of the eligible patients had been tested for these critical driver mutations.
Limitations Small sample size
Conclusion Despite current recommendations to test patients with advanced nsNSCLC for EGFR mutations and ALK rearrangements and physician assertions that they deemed mutation testing essential, fewer than 50% of the patients at the 3 hospitals had been assayed. Our findings imply that large biopsy samples, such as those from surgical or core biopsies, are better than small samples, such as those from needle aspiration for the purpose of molecular testing. In addition, the prevalence of driver mutations among patients who were treated at the cancer center is lower than that published in the literature.
Accepted for publication January 9, 2017
Correspondence Rohit Rao, MD; rohit.rao@ahn.org
Disclosures The authors report no disclosures/conflicts of interest.
Citation JCSO 2017;15(3):e147-e154
©2017 Frontline Medical Communications
doi https://doi.org/10.12788/jcso.0326
Related article
Molecular monitoring and minimal residual disease in the management of chronic myelogenous leukemia
Submit a paper here
EGFR analysis had been conducted at Integrated Oncology, using formalin-fixed, paraffin-embedded tissue. Genomic DNA was isolated, and EGFR mutation analysis was performed using SNaPShot multiplex PCR, primer extension assay for exons 18-21; samples with >4mm2 and ≥50% tumor content were preferred. Macrodissection was used to enrich for tumor cells when samples had lower tumor cellularity and content. ALK rearrangements were tested in the hospital using the Vysis ALK Break Apart FISH probe kit (Abott Molecular Inc, Des Plaines, IL).
We conducted a web-based, 20-question survey about molecular profiling among 110 practitioners to gauge their knowledge and opinions about molecular testing. The practitioners included medical oncologists, thoracic surgeons, pulmonologists, and interventional radiologists. Each received an initial e-mail informing them of the study, inviting them to complete survey, and providing a link to it, and two reminder e-mails at biweekly intervals to maximize survey participation and responses. The questions were aimed at understanding the challenges surrounding molecular testing within our network. Apart from the questions gathering demographic information about the respondents, the questions were intended to highlight the disparities between guideline recommendations and physician practices; to gauge the perceived importance of molecular evaluation; to identify individual, subspecialty, and hospital-based challenges; and to assess physician attitudes toward alternatives to traditional tissue-based testing (Table 1, p. e150). Nineteen of the questions were structured as single or best answer, whereas Question 9, which was aimed at identifying system-based challenges, allowed for multiple answer selections.
Results
There were a total of 206 cases of advanced stage IIIb or IV nsNSCLC identified at three hospitals during 2011-2013. Of those 206 cases, 161 (78.2%) were recorded at the two large academic medical centers, and 45 (21.9%) were recorded at the smaller community-based hospital. Of the total, there were 145 (70.4%) large biopsy specimens and 61 (29.6%) small biopsy specimens. We found that 89 of the 206 cases (43.2 %) had been tested for EGFR mutations, and 49 (23.8%) had been tested for ALK rearrangements (Figure, A and C). In all, 70 (48.3%) large-sample biopsies and 19 (31.1%) small-sample biopsies were submitted for EGFR analysis (Figure, B), and 42 (29%) large-sample biopsies and 7 (11.5%) small-sample biopsies were tested for ALK rearrangements (Figure, D). Large-sample biopsies were more likely to be analyzed for EGFR mutations and ALK rearrangements, with the results reaching statistical significance (P = .023 and P = .007, respectively). Across all samples, a total of 7 EGFR mutations and 1 ALK rearrangement were identified, yielding a prevalence of 7.9% and 2% respectively (Figure, A and C).
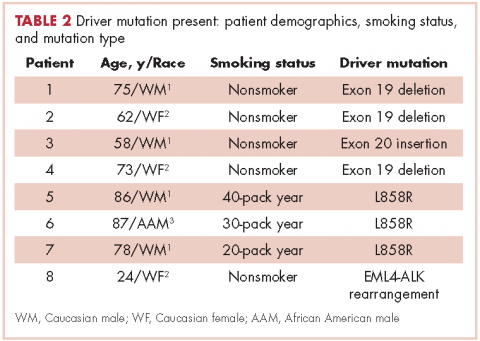
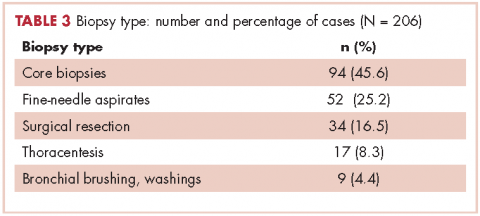
Table 2 shows the demographics, smoking status and type of driver mutation identified. Core biopsies were obtained in 45.6% of the cases and fine-needle aspiration biopsies were obtained in 25.2% of the cases with surgical resections, with thoracentesis and bronchial washings comprising the rest of the biopsies (Table 3).
The average age at diagnosis of the patients in the cases that were analyzed was 69.3 years. Most of the patients (83.9%) identified as white, 3.8% were African American, and 12.6% were in the Unknown category. Of the total number of patients, 11 were identified as never-smokers (5.3%), 50 (24.3%) had a 1-15 pack-year smoking history, 104 (50.5%) had a 16-45 pack-year smoking history, and 41 (19.9%) had a >45 pack-year smoking history.
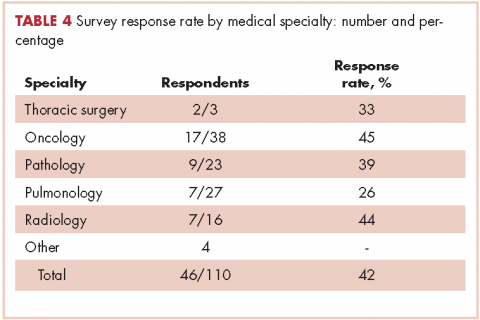
In regard to the survey, 46 of the 110 physicians asked to participate in the survey responded, representing a response rate of 41.8% (range across medical specialties, 26%-45%, Table 4). Of those respondents, 38 (82.6%) indicated they believed molecular evaluation was a very important aspect of NSCLC care, with the remainder indicating it was somewhat important. 91.4% of the respondents who routinely ordered molecular testing agreed that stage IIIb or IV nsNSCLC should undergo molecular evaluation.
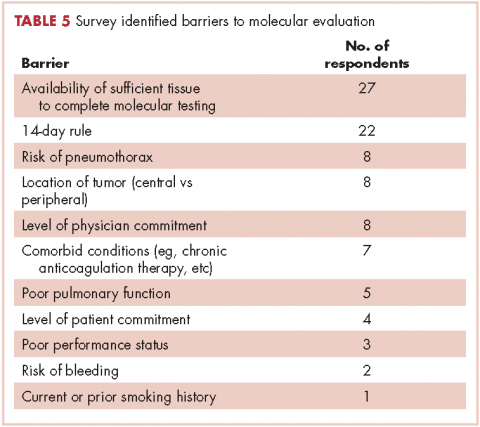
The top barriers to molecular evaluation identified through this survey were the availability of sufficient tissue to complete molecular testing and the Center for Medicare and Medicaid Services’s (CMS’s) 14-day rule that requires hospitals to wait 14 days after the patient is discharged for the lab to receive reimbursement for molecular testing (Table 5).
Discussion
The treatment of advanced nsNSCLC has evolved significantly over the past decade. Molecular profiling is now an essential part of initial evaluation, and larger-sample biopsies are needed to ensure accurate evaluation and appropriate treatment. The detection of EGFR and EML4-ALK driver mutations are associated with increased response to tyrosine kinase inhibitors and are associated with improvement in progression-free survival, patient quality of life, and even overall survival in some studies.12,22,23,24 Early identification of these driver mutations is crucial, however, preliminary observation in our network suggested that a large percentage of patients with advanced nsNSCLC in were not being appropriately evaluated for those mutations. To evaluate our molecular profiling rates, we conducted a retrospective study and reviewed 3 years of registry data at 3 hospitals within our health system. Two of the hospitals included in our analysis were large tertiary academic centers, and one was a community hospital. Our findings confirmed that a large percentage of our patients who are eligible for molecular evaluation are not tested: 56.7% of cases were not tested for EGFR mutations, and 76.2% of cases were not tested for ALK rearrangements.