Distress management in cancer patients in Puerto Rico
This article documents the process of developing and implementing distress management at HIMA-San Pablo Oncologic Hospital in Caguas, Puerto Rico, and summarizes the results of a pilot study to validate the Patient Health Questionnaire (PHQ-9) as a measure to improve the process of emotional distress management in particular. The information is illustrative and useful for medical institutions looking to comply with the American College of Surgeons' Commission on Cancer accreditation standard #3.2, regarding psychosocial distress screening.
Accepted December 20, 2016. Correspondence Maricarmen Ramírez-Solá; mariramirez@himapr.com. Disclosures The author reports no disclosures or conflicts of interest.
JCSO 2017;15(2):68-73. ©2017 Frontline Medical Communications. doi: https://doi.org/10.12788/jcso.0321.
The throughput phase
Within the throughput phase of information organization and analysis, an inventory of health professionals and other in-house consultants needed for distress management was completed. Roles and procedures for information sharing were determined, and we established collaborative agreements with professionals in the community who could contribute to distress management. Members of the psychosocial team held workshops to discuss elements of NCCN guidelines for distress management and to create an action plan for the implementation of the protocol. Data analyses were performed to create a demographic profile of the oncology population at the hospital and assess patient willingness to receive emotional support services,16 which led to the implementation of support group meetings at which additional substantive information was collected about issues affecting cancer patients’ emotions.
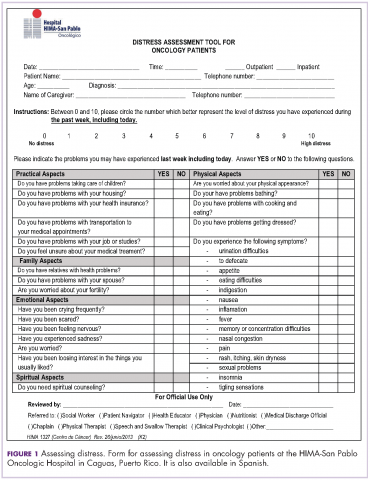
The NCCN Distress Thermometer for measuring distress was translated to Spanish. Its format was adapted, and it was identified as a distress screening tool (DST), which we named Distress Assessment Tool for Oncology Patients (Figure 1). The instrument helps for rapid screening of patient needs and proper determination of initial interventions. In addition, psychometric properties of several instruments were reviewed for instances when patient emotional distress could not be clearly determined. We decided to proceed with the validation of the 9-item Patient Health Questionnaire (PHQ-9) to estimate patient depression level. A proposal for content validation of the PHQ-9 was approved by the University of Puerto Rico institutional review board, and patients were recruited to participate in the pilot study.
The PHQ-9. The PHQ-9 is a self-report version of the PRIME-MD instrument developed to assess mental disorders in clinical settings. It is based on DSM-IV diagnostic criteria.17 The PHQ-9 is the depression module with nine depression symptoms to check off if they become the cause of emotional impairment. Respondents categorized depression symptoms in four frequency degrees representing numeric values: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day). Measures of depression severity are subsequently determined in a Likert-type scale according to numeric calculations of responses: 0-4 (none severe depression), 5-9 (mild), 10-14 (moderate), 15-19 (moderately severe), and 20-27 (severe-major depression).
The instrument is widely used because of its validity in small and large populations. It showed adequate reliability and validity in a small sample of head and neck cancer patients, with a Cronbach’s alpha of 0.80 and a correlation coefficient of 0.71.18 Similarly, it showed good performance in identifying major depression in 4264 cancer outpatients, with sensitivity of 93%, specificity of 81%, and a positive predictive value (PPV) of 25% and negative predictive value (NPV) of 99%.19 Even when administered on a touch screen computer, the instrument showed valid data of depression from patients in treatment.20
The Beck Depression Inventory. We used the Beck Depression Inventory (BDI-II) Spanish version as the gold standard measure for the validation study. It is a 22-items inventory that measures attitudes and symptoms of depression.21 It can be administered in 10 minutes and has shown good psychometric measures when administered in Spain and Puerto Rico.22, 23
The pilot study. In all, 44 cancer patients who were receiving outpatient treatment at the radiotherapy unit agreed to participate in the study. The participants signed a consent form after the confidentiality protection measures and the main objectives of the study had been explained to them. Patients were interviewed individually during November and December 2012, with the Spanish versions of the PHQ-9 and BDI-II administered by one of two interviewers. At the beginning of each interview, the patient was asked 10 questions so that we could gather demographic data and confirm participant eligibility: aged 21 years or older, born and raised in Puerto Rico, being a Spanish speaker, and having a primary cancer diagnosis with no previous disease. Three patients were excluded from the sample because they either had cancer previously or had a recurrence or metastasis. The final sample consisted of 41 outpatients (N = 41).
Data analysis for demographics was completed with STATA v.12 software. Measures of central tendency and dispersion as well as PHQ-9 internal consistency analysis were made through Cronbach alpha with SPSS.
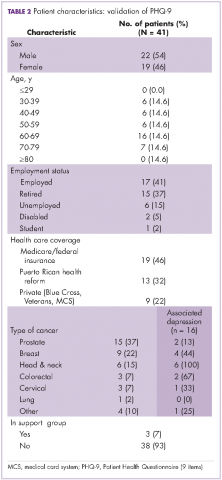
From a total of 41 patients surveyed, 22 (54%) were men and 19 (46%) were women, with an overall median age of 61 years. Among the men, 15 (68%) had a prostate cancer diagnosis and among women, 9 (47.4%) had a breast cancer diagnosis. In regard to health insurance, 19 (46%) had Medicare or Veterans/federal insurance coverage, and 13 (32%) had Reforma, the Puerto Rican government health insurance program partially funded by Medicaid funds. In addition, 8 participants (20%) were unemployed or disabled. As previously stated, all of the patients were in ambulatory care. Only 3 (7%) were participating in support groups.
Of all the respondents, 16 (39%) reported some level of depression. In particular, 2 (5%) showed severe-major depression, 4 (10%) moderately severe depression, and 10 (24%) moderate depression. Of those with depression, 8 (50%) were women, 8 (50%) were men. All 6 of the patients with head and neck cancer showed moderate or moderately severe depression (Table 2).
When respondent PHQ-9 scoring reflected moderate to severe depression (>10), a letter was sent to the patient’s radio-oncologist for referral to counseling and clinical psychological evaluation. All participants had access to the support group program, to a radiotherapy education program meeting weekly, and written information about their cancer diagnosis and treatment. They also were interviewed by the psychosocial coordinator or patient navigator for further assessment.