Rifampin for Prosthetic Joint Infections: Lessons Learned Over 20 Years at a VA Medical Center
Background: The Minneapolis Veterans Affairs Health Care System uses debridement and implant retention (DAIR) combined with oral rifampin and a second antibiotic to treat orthopedic implant infections. However, the success rate of this approach in a veteran population is unknown.
Methods: We performed a retrospective analysis of patients who underwent DAIR with a rifampin-containing regimen for an orthopedic implant infection over the past 20 years at the Minneapolis Veterans Affairs Health Care System. The primary outcome was treatment success among participants who were treated with curative intent, defined as planned device retention without ongoing antibiotic use. Secondary outcomes were treatment harms and therapy duration. Treatment success was defined as the absence of recurrent infection or further measures to suppress infection within 1 year of completing antimicrobial therapy.
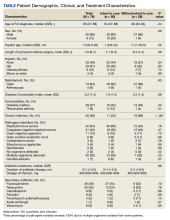
Results: A total of 78 patients (88% male) were included (median age, 65.5 years), with 50 treated with curative intent (primary analysis group). Forty-one participants (82%) in the curative intent group experienced treatment success. The success rate was higher among participants whose implant was < 2 months old vs those whose implant was ≥ 2 months old (93% vs 65%, respectively; P = .02). The 28 participants treated without curative intent had more comorbidities, higher rates of chronic infection, and older implants than those treated with curative intent.
Conclusions: Veterans with orthopedic implant infections can be successfully treated with DAIR combined with a rifampin-containing antimicrobial regimen. Success is highest for patients with a recent implant. Debridement and implant retention using regimens that include rifampin is an evidence-based strategy for managing patients with infected prosthetic hardware. Here we report that this approach is feasible in a veteran population, especially with recently implanted prosthetic material.
Treatment Outcomes
The primary outcome was treatment success, defined as meeting all of the following: (1) lack of clinical signs and symptoms of infection; (2) absence of radiological signs of loosening or infection within 1 year after the conclusion of treatment; and (3) absence of additional PJI treatment interventions for the prosthesis of concern within 1 year after completing the original antibiotic treatment.
Treatment failure was defined as meeting any of the following: (1) recurrence of PJI (original strain or different microorganism) within 1 year after the completion of antibiotic therapy; (2) death attributed to PJI anytime after the initial debridement; (3) removal of the prosthetic joint within 1 year after the completion of antibiotic therapy; or (4) long-term antibiotic use to suppress the PJI after the completion of the initial antibiotic therapy.
Statistical Analysis
Descriptive statistics were used to define the baseline characteristics of patients receiving rifampin therapy for orthopedic implant infections at the MVAHCS. Variables analyzed were age, sex, race and ethnicity, type of implant, age of implant, duration of symptoms, comorbidities (diabetes and rheumatoid arthritis), and presence of chronic infection. Patients were classified as having a chronic infection if they received previous infection treatment (antibiotics or surgery) for the orthopedic device in question. We created this category because patients with persistent infection after a medical or surgical attempt at treatment are likely to have a higher probability of treatment failure compared with those with no prior therapy. Charlson Comorbidity Index was calculated using clinical information present at the onset of infection.9 Fisher exact test was used to assess differences between categorical variables, and an independent t test was used to assess differences in continuous variables. P < .05 indicated statistical significance.
To assess the ability of a rifampin-based regimen to achieve a cure of PJI, we grouped participants into 2 categories: those with an intent to cure strategy and those without intent to cure based on documentation in the electronic health record (EHR). Participants who were prescribed rifampin with the documented goal of prosthesis retention with no further suppressive antibiotics were included in the intent-to-cure group, the primary focus of this study. Those excluded from the intent-to-cure group were given rifampin and another antibiotic, but there was a documented plan of either ongoing chronic suppression or eventual explantation; these participants were placed in the without-intent-to-cure group. Analysis of treatment success and failure was limited to the intent-to-cure group, whereas both groups were included for assessment of adverse effects (AEs) and treatment duration. This project was reviewed by the MVAHCS Institutional Review Board and determined to be a quality improvement initiative and to not meet the definition of research, and as such did not require review; it was reviewed and approved by the MVAHCS Research and Development Committee.
RESULTS
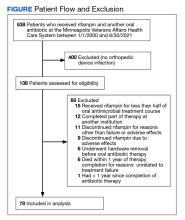
A total of 538 patients were identified who simultaneously received rifampin and another oral antibiotic between January 1, 2000, and June 30, 2021.