Weight Gain in Veterans Taking Duloxetine, Pregabalin, or Both for the Treatment of Neuropathy
Introduction: Peripheral neuropathy is a common condition with an estimated incidence of 3 million cases in the United States per year, with manifestations including weakness, numbness, burning or tingling sensations, and lingering pain. The burden of neuropathy may be greater among veterans due to the higher prevalence of type 2 diabetes mellitus (T2DM) and an aging population. Among the medications used to treat neuropathy are duloxetine and pregabalin. It has been observed at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS) that veterans who are treated for neuropathy with duloxetine, pregabalin, or both, may experience significant weight gain after starting therapy. The purpose of this study was to evaluate the association of weight gain in veterans taking duloxetine, pregabalin, or both, for the treatment of neuropathy.
Methods: This was a retrospective, chart review study conducted at the SFVAHCS. The primary end point of this study was the change in body weight, expressed in pounds, after 12 to 18 months of treatment. The secondary end points of this study were the percent change in body weight; duration effect; dose effect, which evaluated weight gain at doses of duloxetine > 60 mg/d and pregabalin at doses > 300 mg/d; change in hemoglobin A 1c in patients with prediabetes and T2DM, and involvement in the Managing Overweight Veterans Everywhere (MOVE!) weight management program.
Results: The change in body weight after 12 to 18 months of treatment was -0.8 lb in the duloxetine group, +2.9 lb in the pregabalin group, and +5.5 lb in the pregabalin plus duloxetine group ( P = .12). The change in body weight after > 12 months of treatment was -0.88 lb in the duloxetine group, +3.6 lb in the pregabalin group, and +8.5 lb in the duloxetine plus pregabalin group ( P = .046). The change in body weight in patients who received increased doses of the study agents was -2.8 lb in the duloxetine group and +6.5 lb in the pregabalin group ( P = .047).
Conclusions: There was no significant difference in weight in veterans who took duloxetine, pregabalin, or both, for treatment of neuropathy after 12 to 18 months of therapy. However, there was a difference in weight gain among the 3 groups when therapy lasted > 12 months. The combination therapy of pregabalin and duloxetine was associated with the most amount of weight gain, followed by pregabalin alone. Monotherapy of duloxetine had minimal association with weight gain. In veterans who took increased doses of duloxetine or pregabalin, there was a difference in weight between the monotherapy groups, with pregabalin associated with weight gain and duloxetine associated with weight loss.
Methods
A retrospective, single-center, chart review was conducted at the Sioux Falls Veterans Affairs Health Care System (SFVAHCS). Data were collected through manual chart review of US Department of Veterans Affairs (VA) electronic health records (EHRs). Patients included were veterans aged 18 to 89 years who were initiated on duloxetine and/or pregabalin between October 2015 and September 2018.
The primary end point of this study was the change in body weight, expressed in pounds, after 12 to 18 months of treatment. If multiple weights were obtained during the 12- to 18-month period, the weight recorded closest to 12 months was used. The secondary end points included the percent change in body weight and dose effect, which evaluated change in weight at doses of duloxetine > 60 mg/d, and pregabalin at doses > 300 mg/d. Duration of effect was evaluated as a secondary end point; contrary to the primary end point, the weight furthest from 12 months was recorded. The change in hemoglobin A1c (HbA1c) in patients with prediabetes and DM also was investigated as a secondary end point. Last, involvement in the Managing Overweight Veterans Everywhere (MOVE!) weight management program at SFVAHCS and its effect on weight gain was reviewed.
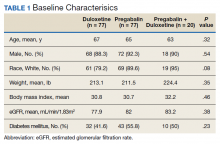
Baseline characteristics were collected to determine the variability between each study group. Data collected during the study included age, sex, race, weight, BMI, HbA1c, eGFR, DM diagnosis, insulin therapy prescription, duration of use, and MOVE! program participation.
Statistical Analysis
The primary and secondary end points were analyzed using an analysis of variance statistical test. Results were considered statistically significant at P < .05.
Results
A total of 174 participants were included in this study, with 77 in each monotherapy group, and 22 in the combination therapy group. More than 300 patients were excluded from the study due to prespecified inclusion and exclusion criteria. Baseline characteristics were similar among the 3 groups, with no statistically significant differences identified (Table 1).