Guideline Concordance with Durvalumab in Unresectable Stage III Non-Small Cell Lung Cancer: A Single Center Veterans Hospital Experience
Background: Durvalumab is recommended by national guidelines for patients with unresectable stage III non-small cell lung cancer (NSCLC) following concurrent chemoradiation therapy (CRT). Nonadherence to guidelines is associated with adverse outcomes. We studied the adherence and identified barriers to durvalumab usage at the Birmingham Veterans Affairs Medical Center (VAMC) Oncology Clinic in Alabama.
Methods: Using retrospective analysis, we assessed the use of consolidative durvalumab among veterans at Birmingham VAMC. The health records of all veterans with stage III unresectable NSCLC from October 2017 to August 2019 were reviewed. Data collected included demographics, barriers to CRT initiation and completion, durvalumab usage, and reasons for not prescribing durvalumab.
Results: In our data review, 34 patients were found to have stage III unresectable NSCLC. Twenty (58.8%) of those 34 initiated CRT, but only 16 (47.1%) completed CRT treatment and 7 (20.6%) underwent further treatment with durvalumab. Of the 14 patients who did not initiate CRT, the most common reasons were poor performance status and/or the presence of comorbidities. Of the evaluable cohort of 34, 11 (32.4%) patients with stage III NSCLC received durvalumab. Of the 9 eligible patients who did not receive durvalumab, the most common reasons cited were toxicities experienced during or following CRT (11.8%).
Conclusions: Just one-third of patients were eligible to receive durvalumab at Birmingham VAMC. This was likely due to the difference between clinical trial and real-world patient populations. Interventions to address socioeconomic and system level barriers to improve our center’s delivery of lung cancer treatment are planned.
Methods
The Birmingham VAMC Outpatient Oncology Clinic billing data identified all individuals diagnosed with lung cancer treated between October 2017 and August 2019. Patients who did not have NSCLC that was stage III and unresectable were excluded from our study. Patients who did not receive a majority of their treatment at US Department of Veterans Affairs (VA) facilities were excluded as well. Each patient’s demographic, functional level, and tumor characteristics during the treatment planning phase and follow-up visits were obtained. Two investigators who evaluated health care provider documentation using the VA Computerized Patient Record System (CPRS) conducted chart reviews.
The primary outcomes were the proportion of patients who received concurrent CRT and the proportion who received durvalumab consolidation. Our chart review also categorized reasons for nonreceipt of concurrent CRT and subsequent durvalumab. Documented reasons for guideline discordancy were generated empirically and broadly. We noted if documentation was unclear and included reasons for why a veteran was not a candidate for CRT, the presence of toxicities associated with CRT, and a patient’s refusal for therapy despite medical advice. Descriptive data were analyzed for all clinical or demographic characteristics and outcomes.
This was considered an internal quality improvement initiative. As such, Birmingham VAMC did not require institutional review board approval for the study. The facility is accredited by the American College of Surgeons Commission on Cancer.
Results
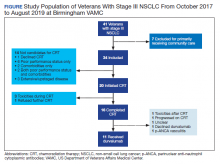
A total of 41 veterans with stage III NSCLC were identified to have established care in the Birmingham VAMC Oncology Clinic between October 2017 and August 2019. Of these, 7 received the majority of their treatment from community-based non-VA facilities and 14 were not candidates for CRT and were excluded from this study.
The mean (SD) age of study participants was 70.0 (8.4) years (range, 57 to 92 years). Most of the study veterans (33; 97.1%) were male and 20 (58.8%) were African American (Table). Eighteen (53%) of study participants had clinical stage IIIa NSCLC; 19 (56%) showed a squamous subtype of NSCLC. A majority (53%) of the veterans studied were evaluated to be functionally fit with an ECOG status of 0 to 1, although documentation of ECOG status was lacking in 5 (14.7%) patients in the initial treatment planning visit records. It was unclear if performance status had been reevaluated and changes noted over the course of concurrent CRT.
CRT Patients
The relative distribution of veterans who underwent CRT for stage III NSCLC plus the reasons they did not receive guideline-based treatment with durvalumab is shown in the Figure. Fourteen patients (41%) were inappropriate candidates for CRT; the most common reason for this was their poor performance status upon initial evaluation and 3 patients (8.8%) in the study had extensive disease or were upstaged upon follow-up clinic visit.
Twenty (59%) veterans in the study initiated CRT. However, only 16 (47.1%) completed CRT. Those who dropped out of CRT did so because of toxicities that included various cytopenia, gastrointestinal toxicities due to radiation and/or chemotherapy, or failure to thrive.