Enoxaparin vs Continuous Heparin for Periprocedural Bridging in Patients With Atrial Fibrillation and Advanced Chronic Kidney Disease
Economic Analysis
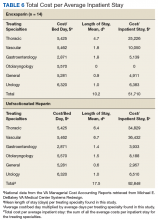
The average cost differences (Table 6) of hospitalization between enoxaparin and UFH were calculated using the average LOS per treating specialty multiplied by the national average cost of the MCO for an inpatient bed day in 2018.12 The treating specialty with the longest average LOS in the enoxaparin arm was thoracic (4.7 days). The UFH arm also had a large LOS (average days) for the thoracic specialty (6.4 days); however, the vascular specialty (6.7 days) had the longest average LOS in this group. Due to a mean LOS of 10.2 days in the enoxaparin arm, which was further stratified by treating specialty, the total cost per average inpatient stay was calculated as $51,710. On the other hand, patients in the UFH arm had a total cost per average inpatient stay of $92,848.
Monitoring
Anti-factor Xa levels for LMWH monitoring were not analyzed in this study due to a lack of values collected; only 1 patient had an anti-factor Xa level checked during this time frame. Infusion rates of UFH were adjusted based on aPTT levels collected per MEDVAMC inpatient anticoagulation protocol. The average percentage of aPTT in therapeutic range was 46.3% and the mean time-to-therapeutic range (SD) was about 2.4 (1.3) days. Due to this study’s retrospective nature, there were inconsistencies with availability of documentation of UFH infusion rates. For this reason, these values were not analyzed further.
Discussion
In 2017, the American College of Cardiology published the Periprocedural Anticoagulation Expert Consensus Pathway, which recommends for patients with AF at low risk (CHA2DS2VASc 1-4) of thromboembolism to not be bridged (unless patient had a prior VTE or stroke/TIA).13 Nearly half the patients in this study, were classified as moderate-to-high thrombotic risk as evidenced by a CHA2DS2VASc > 4 with a mean score of 4.8. Due to this study’s retrospective design from 2008 to 2017, many of the clinicians may have referenced the 2008 CHEST antithrombotic guidelines when making the decision to bridge patients; these guidelines and the previous MEDVAMC anticoagulation protocol recommend bridging patients with AF with CHADS2 > 2 (moderate-to-high thrombotic risk) in which all but 1 of the patients in this study met criteria.1,14 In contrast to the landmark BRIDGE trial, the mean CHADS2 score in this study was 3.6; this is an indication that our patient population was of individuals at an increased risk of stroke and embolism.