COVID-19 therapy: What works? What doesn’t? And what’s on the horizon?
Here is the latest evidence on the efficacy and safety of treatments that are FDA approved or authorized, in clinical trials, or not recommended to combat COVID-19.
PRACTICE RECOMMENDATIONS
› Use antivirals (eg, molnupiravir, nirmatrelvir packaged with ritonavir [Paxlovid], and remdesivir) and monoclonal antibody agents (eg, bebtelovimab) effective against the circulating Omicron variant, to treat symptoms of mild-to-moderate COVID-19 illness. C
› Treat severely ill hospitalized COVID-19 patients who require supplemental oxygen with dexamethasone, alone or in combination with remdesivir, to produce better outcomes. B
› Consider administering baricitinib or tocilizumab, in addition to dexamethasone with or without remdesivir, to COVID-19 patients with rapidly increasing oxygen requirements. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Outpatient treatment
Several assessment tools that take into account patients’ age, respiratory status, and comorbidities are available for triage of patients infected with COVID-19.8
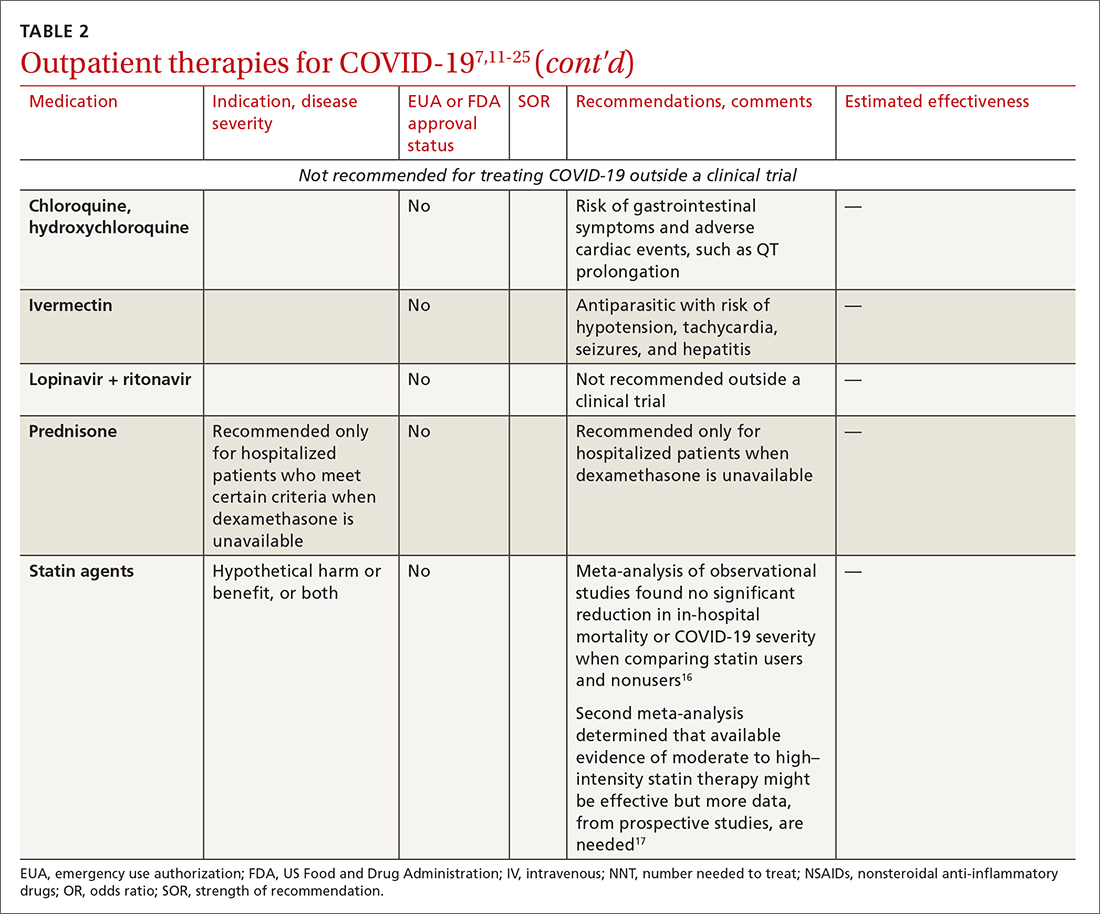
Most (> 80%) patients with COVID-19 have mild infection and are safely managed as outpatients or at home.9,10 For patients at high risk of severe disease, a few options are recommended for patients who do not require hospitalization or supplemental oxygen; guidelines on treatment of COVID-19 in outpatient settings that have been developed by various organizations are summarized in TABLE 2.7,11-25
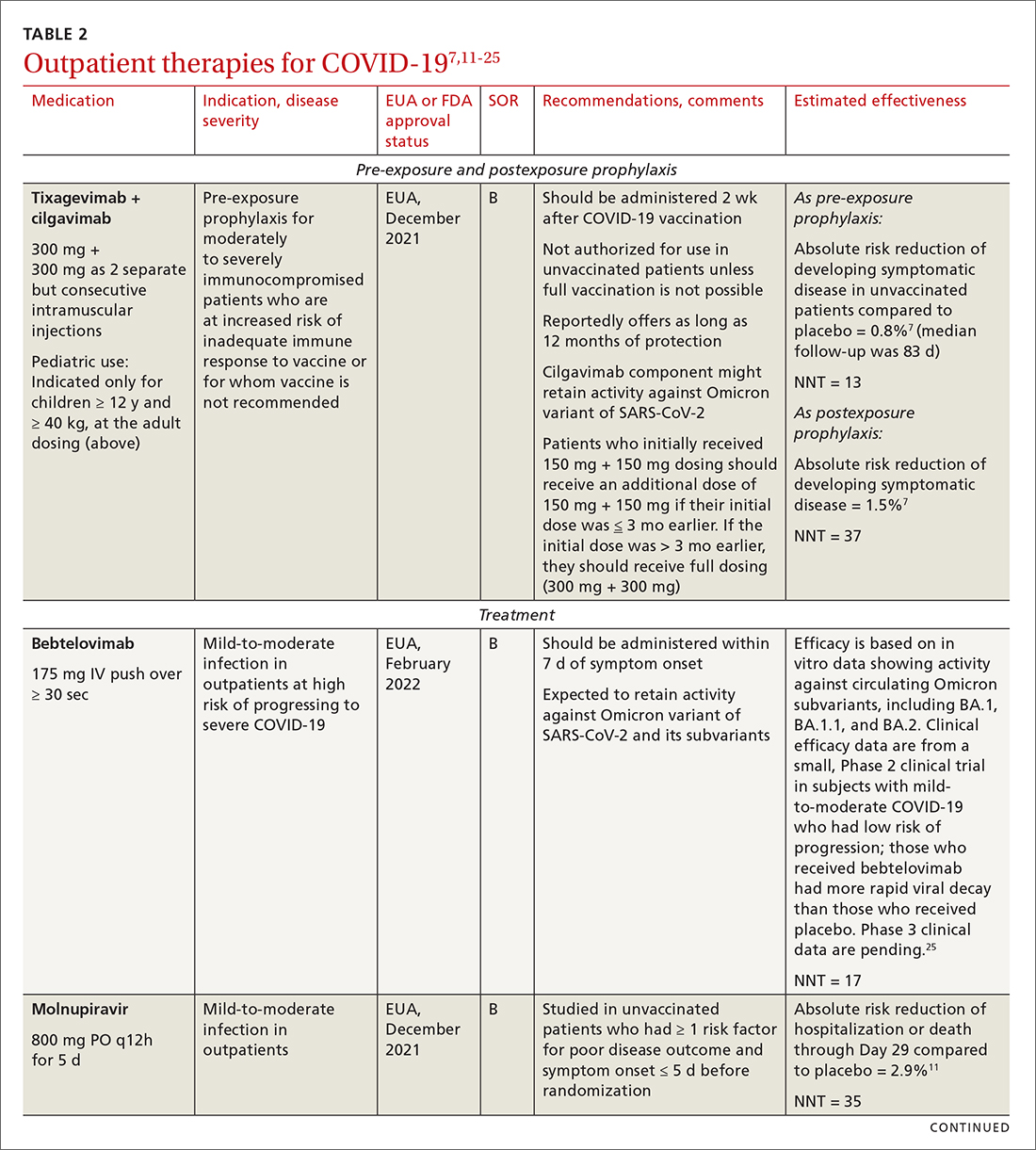
Antiviral drugs target different stages of the SARS-CoV-2 replication cycle. They should be used early in the course of infection, particularly in patients at high risk of severe disease.
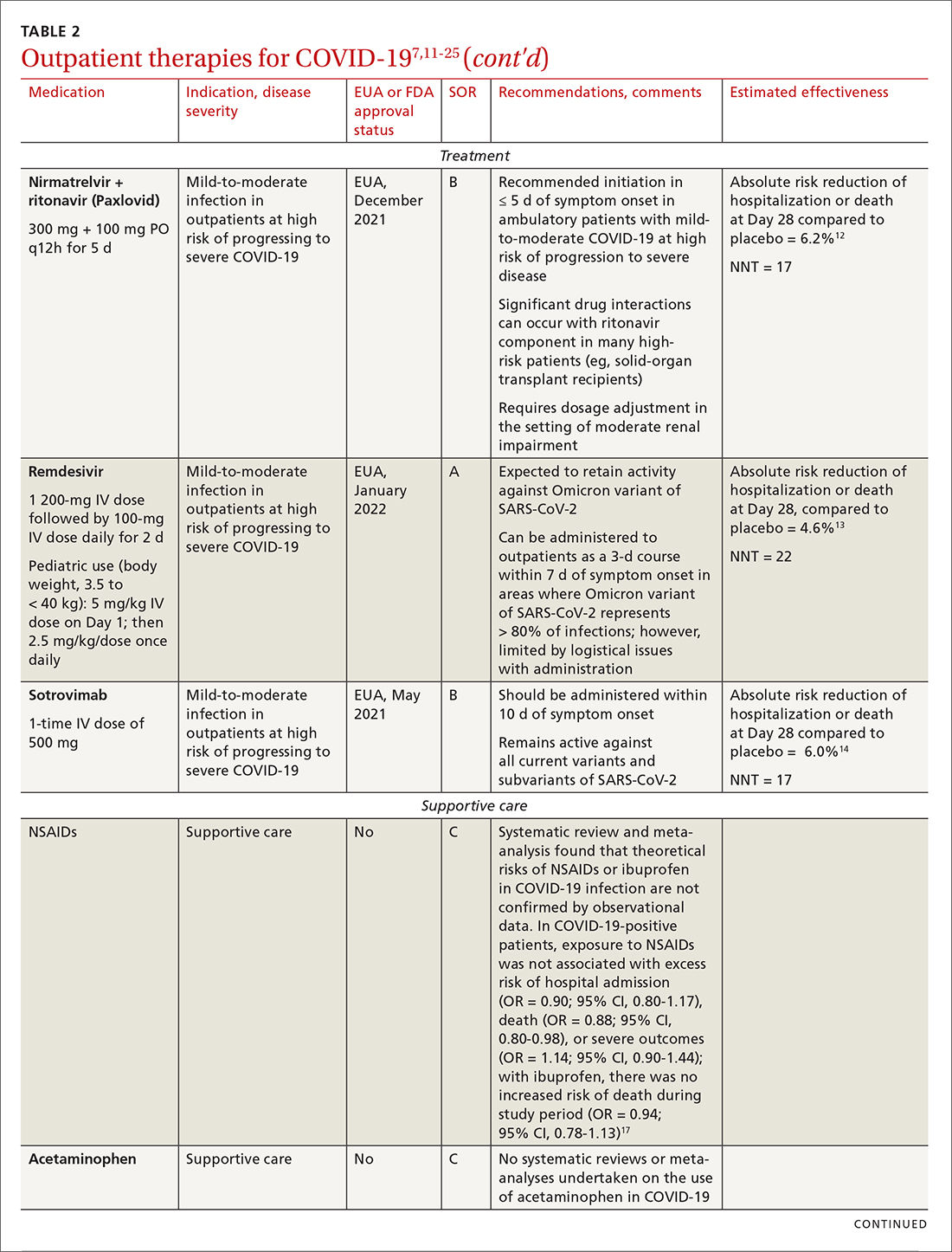
IDSA recommends antiviral therapy with molnupiravir, nirmatrelvir + ritonavir packaged together (Paxlovid), or remdesivir.11,12,26,27 Remdesivir requires intravenous (IV) infusion on 3 consecutive days, which can be difficult in some clinic settings.13,28 Nirmatrelvir + ritonavir should be initiated within 5 days after symptom onset. Overall, for most patients, nirmatrelvir + ritonavir is preferred because of oral dosing and higher efficacy in comparison to other antivirals. With nirmatrelvir + ritonavir, carefully consider drug–drug interactions and the need to adjust dosing in the presence of renal disease.28,29 There are no data on the efficacy of any combination treatments with these agents (other than co-packaged Paxlovid).
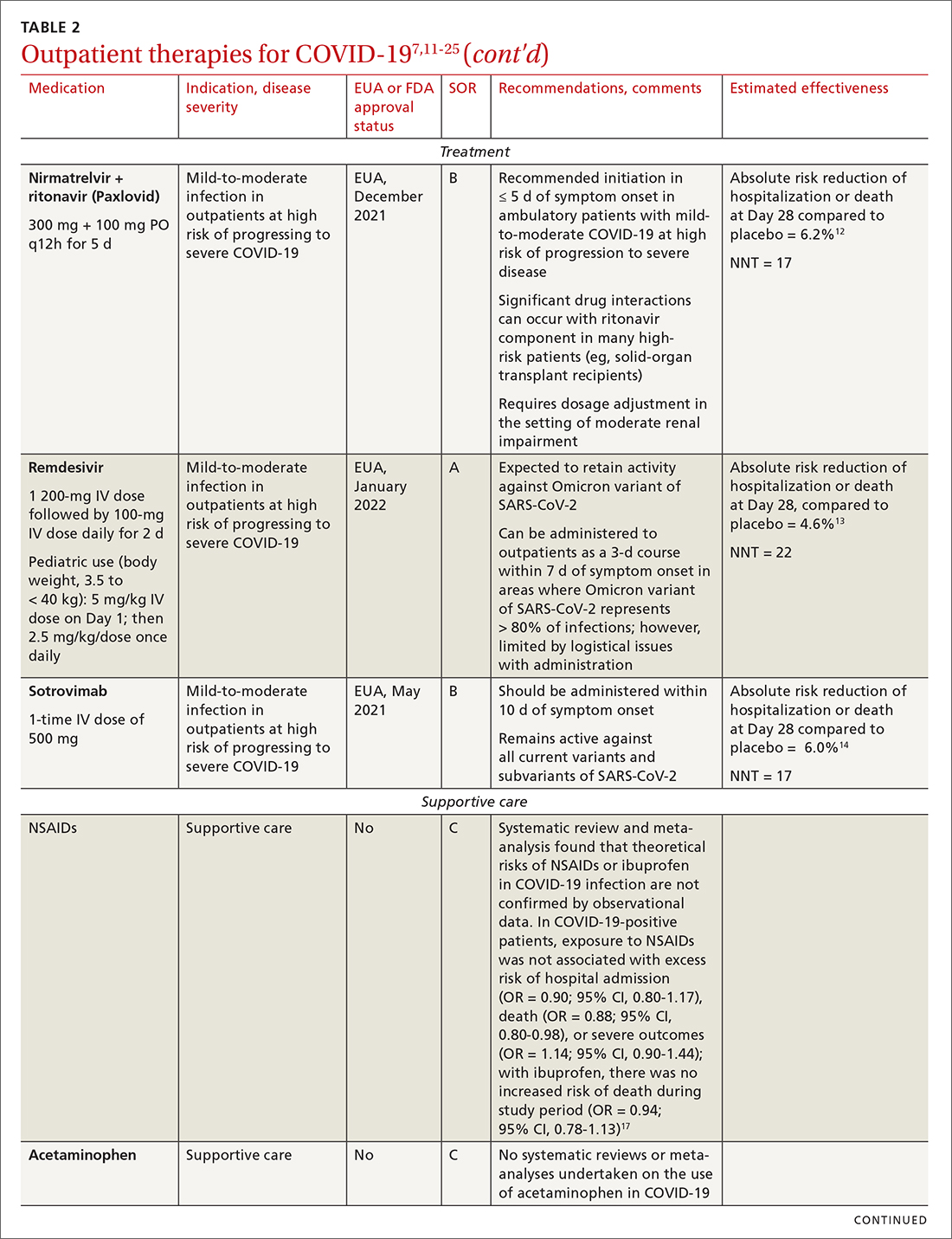
Monoclonal antibodies for COVID-19 are given primarily intravenously. They bind to the viral spike protein, thus preventing SARS-CoV-2 from attaching to and entering cells. Bamlanivimab + etesevimab and bebtelovimab are available under an EUA for outpatient treatment.14b Treatment should be initiated as early as possible in the course of infection—ideally, within 7 to 10 days after onset of symptoms.
Continue to: Bebtelovimab was recently given...






