Postherpetic Isotopic Responses With 3 Simultaneously Occurring Reactions Following Herpes Zoster
Inflammatory, infectious, and neoplastic processes can all occur in prior areas of herpes infection (postherpetic isotopic response [PHIR]). Postzoster granulomatous dermatitis is among the most frequently encountered PHIR, but the exact pathogenesis has not been fully elucidated. Rarely, multiple diseases manifest concurrently in a PHIR. We report a case of cutaneous chronic lymphocytic leukemia (CLL) with an associated granulomatous dermatitis and medium-vessel vasculitis occurring simultaneously at the site of prior herpes zoster. Clinicians and dermatopathologists should be aware of the multiplicity of postzoster isotopic responses and should consider the possibility of multiple diseases manifesting in the same clinical lesion in this setting.
Practice Points
- Multiple diseases may present in prior sites of herpes infection (postherpetic isotopic response).
- Granulomatous dermatitis is the most common postherpetic isotopic response, but other inflammatory, neoplastic, or infectious conditions also occur.
- Multiple conditions may present simultaneously at sites of herpes infection.
- Cutaneous involvement by chronic lymphocytic leukemia (CLL) can be easily overlooked in this setting.
Postherpetic isotopic response (PHIR) refers to the occurrence of a second disease manifesting at the site of prior herpes infection. Many forms of PHIR have been described (Table), with postzoster granulomatous dermatitis (eg, granuloma annulare, sarcoidosis, granulomatous vasculitis) being the most common.1 Both primary and metastatic malignancies also can occur at the site of a prior herpes infection. Rarely, multiple types of PHIRs occur simultaneously. We report a case of 3 simultaneously occurring postzoster isotopic responses--granulomatous dermatitis, vasculitis, and chronic lymphocytic leukemia (CLL)--and review the various types of PHIRs.
Case Report
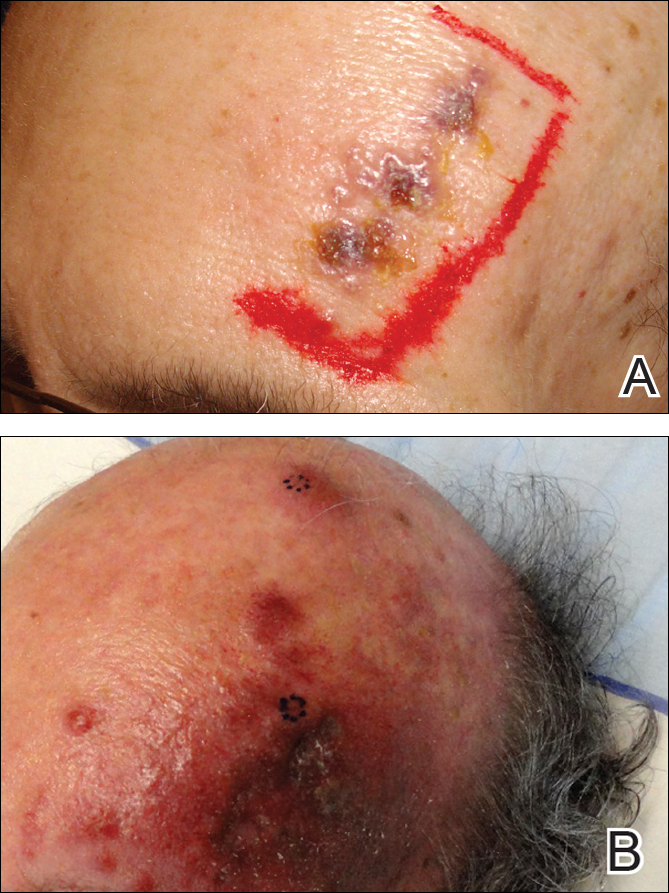
A 55-year-old man with a 4-year history of CLL was admitted to the hospital due to a painful rash on the left side of the face of 2 months' duration. Erythematous to violaceous plaques with surrounding papules and nodules were present on the left side of the forehead and frontal scalp with focal ulceration. Two months prior, the patient had unilateral vesicular lesions in the same distribution (Figure 1A). He initially received a 3-week course of acyclovir for a presumed herpes zoster infection and showed prompt improvement in the vesicular lesions. After resolution of the vesicles, papules and nodules began developing in the prior vesicular areas and he was treated with another course of acyclovir with the addition of clindamycin. When the lesions continued to progress and spread down the left side of the forehead and upper eyelid (Figure 1B), he was admitted to the hospital and assessed by the consultative dermatology team. No fevers, chills, or other systemic symptoms were reported.
,
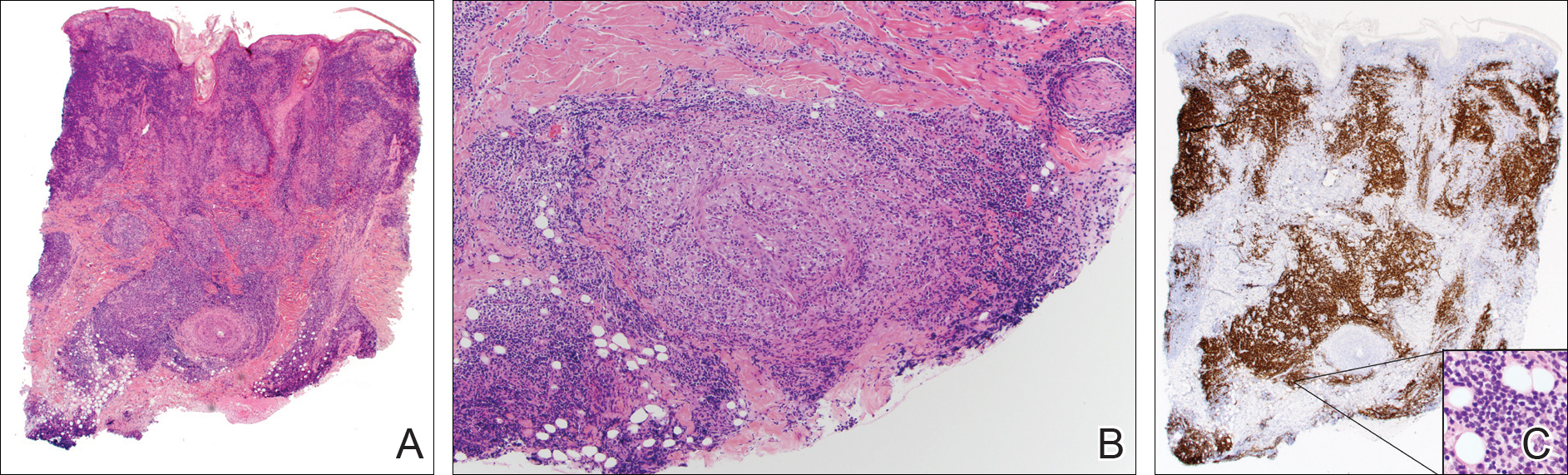
A punch biopsy showed a diffuse lymphocytic infiltrate filling the dermis and extending into the subcutis with nodular collections of histiocytes and some plasma cells scattered throughout (Figure 2A). A medium-vessel vasculitis was present with numerous histiocytes and lymphocytes infiltrating the muscular wall of a blood vessel in the subcutis (Figure 2B). CD3 and CD20 immunostaining showed an overwhelming majority of B cells, some with enlarged atypical nuclei and a smaller number of reactive T lymphocytes (Figure 2C). CD5 and CD43 were diffusely positive in the B cells, confirming the diagnosis of cutaneous CLL. CD23 staining was focally positive. Immunostaining for κ and λ light chains showed a marginal κ predominance. An additional biopsy for tissue culture was negative. A diagnosis of postzoster granulomatous dermatitis with vasculitis and cutaneous CLL was rendered.