Physician Skin Examinations for Melanoma Screening
A variety of estimates of the value and impact of physician skin examinations (PSEs) in screening for melanoma have been published. Although current melanoma screening guidelines vary, new evidence supports improved melanoma outcomes associated with PSEs. In this systematic review, we evaluated 5 observational studies of the impact of PSEs on melanoma thickness at diagnosis and melanoma mortality rates. Although definitive evidence from randomized controlled trials supporting improved health outcomes associated with PSEs is lacking, these well-designed observational studies have found PSEs to be correlated with thinner melanomas at diagnosis and reduced melanoma mortality rates.
Practice Points
- Current guidelines regarding melanoma screening are inconsistent.
- There is a growing pool of evidence supporting screening to improve melanoma outcomes.
Results
A total of 705 titles were screened, 98 abstracts were assessed for eligibility, 42 full-text reviews were carried out, and 5 eligible studies were identified (Figure 1). Five observational studies were included in the final review. A summary of the results is presented in Table 1.
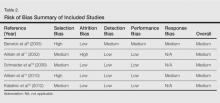
Included studies were assessed for several types of biases, including selection bias, attrition bias, detection bias, performance bias, and response bias. The judgments were given for each domain (Table 2). There was heterogeneity in study design, reporting of total-body skin examination methods, and reporting of outcomes among all 5 studies. All 5 studies were assessed as having a medium risk of bias.
Physician Skin Examination Impact
One article by Berwick et al9 reanalyzed data from a 1996 study10 and provided no significant evidence regarding the benefits of PSEs in the reduction of melanoma mortality. Data for 650 patients with newly diagnosed melanomas were obtained from the Connecticut Tumor Registry, a site for the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, along with 549 age- and sex-frequency matched controls from the general population.10 Participants were followed biannually for a mean of 5.4 years. Of the original 650 case patients, 122 were excluded from the study with reasons provided. Physician skin examination was defined as a positive response to the following questionnaire item: “[Before your recent biopsy] did the doctor examine your skin during any of your visits?”9 Data analysis showed no significant association between PSE and death from melanoma. Upon univariate analysis, the hazard ratio for physician screening was 0.7 (95% confidence interval [CI], 0.4-1.3).9
The SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) project, which was undertaken in Schleswig-Holstein, Germany, is the world’s largest systematic population-based skin cancer screening program.15 The participation rate was 19% (N=360,288) of the eligible population (citizens aged ≥20 years with statutory health insurance). Screening was a 2-step process performed by trained physicians: initial general practitioner whole-body skin examination followed by referral to a dermatologist for evaluation of suspicious skin findings. Five years after the SCREEN program was conducted, melanoma mortality declined by 47% per 100,000 men and by 49% per 100,000 women. The annual percentage change in the most recent 10-year period (2000-2009) was 7.5% (95% CI, –14.0 to –0.5; P<.05) for men and 7.1% for women (95% CI, –10.5 to –2.9; P<.05). Simultaneously, the melanoma mortality rates in the 4 unscreened adjacent regions and the rest of Germany were stable, significantly (P<.05) different from the decline in mortality observed in Schleswig-Holstein.15
A community-based, prospective cohort study investigated the impact of an employee melanoma screening program at the Lawrence Livermore National Laboratory (Livermore, California) (1984-1996) demonstrated an impact on melanoma thickness and mortality rates.12 The cohort (approximately 5100 participants) was followed over 3 phases of surveillance: (1) preawareness (1969-1975), (2) early awareness of increased melanoma risk (1976-1984), and (3) screening program (1984-1996). The screening program encouraged employees to self-examine their skin for “suggestive lesions”; if a suggestive lesion was found, a full-body skin examination was performed by a physician. After being evaluated, participants with melanoma, dysplastic nevi, 50 or more moles, or a family history of melanoma were offered a periodic full-body examination every 3 to 24 months, often with full-body photography and dermoscopy. Physician skin screening resulted in a reduction in crude incidence of thicker melanomas (defined as >0.75 mm) during the 3 study phases. Compared with the early-awareness period (phase 2), a 69% reduction in the diagnosis of thick melanomas was reported in the screening program period (phase 3)(P=.0001). During the screening period, no eligible melanoma deaths occurred in the study population, whereas the expected number of deaths was 3.39 (P=.034) based on observed melanoma mortality in 5 San Francisco/Oakland Bay–area counties in California as reported to the SEER program from 1984 to 1996.12
The strongest evidence for reduced thickness of melanomas detected via PSEs was reported in a population-based, case-control study by Aitken et al14 of all residents in Queensland, Australia, aged 20 to 75 years with a histologically confirmed first primary invasive cutaneous melanoma diagnosed between January 2000 and December 2003. Whole-body PSE in the 3 years before diagnosis was inversely associated with tumor thickness at diagnosis (χ2=44.37; P<.001), including a 14% lower risk of diagnosis of a thick melanoma (>0.75 mm)(odds ratio [OR], 0.86; 95% CI, 0.75-0.98) and a 40% lower risk of diagnosis of a melanoma that was 3 mm or larger (OR, 0.60; 95% CI, 0.43-0.83). The investigators applied melanoma thickness-specific survival estimates to the thickness distribution of the screened and unscreened cases in their sample to estimate melanoma deaths within 5 and 10 years of diagnosis. Compared to the unscreened cases, they estimated that the screened cases would have 26% fewer melanoma deaths within 5 years of diagnosis and 23% fewer deaths within 10 years.14