Why are we doing cardiovascular outcome trials in type 2 diabetes?
ABSTRACTCardiovascular disease is the leading cause of morbidity and death in people with diabetes mellitus. While worsening hyperglycemia is directly associated with poorer outcomes, studies aiming at euglycemia have failed to show an advantage over modest glucose-lowering strategies. Several diabetes drugs that were approved solely on the basis of their glucose-lowering potential were later shown to increase cardiovascular risk.
KEY POINTS
- The worldwide burden of type 2 diabetes is increasing dramatically as obesity rates increase, populations age, and people around the world adopt a Western diet.
- Diabetes increases the risk of atherosclerotic cardiovascular disease, which remains the leading cause of death in diabetic patients.
- Lowering blood glucose alone may not necessarily amount to reduction in adverse cardiovascular events.
- Clinical trials of new drugs for type 2 diabetes must prove cardiovascular safety in addition to glucose-lowering potential before the drugs gain final regulatory approval.
- Aggressive risk factor modification (smoking cessation, control of hypertension, and treatment of hyperlipidemia with statins) remains paramount in reducing cardiovascular risk in people with diabetes.
A 50-year-old man with hypertension presents to the internal medicine clinic. He has been an active smoker for 15 years and smokes 1 pack of cigarettes a day. He was recently diagnosed with type 2 diabetes mellitus after routine blood work revealed his hemoglobin A1c level was elevated at 7.5%. He has no current complaints but is concerned about his future risk of a heart attack or stroke.
THE BURDEN OF DIABETES MELLITUS
The prevalence of diabetes mellitus in US adults (age > 20) has tripled during the last 30 years to 28.9 million, or 12% of the population in this age group.1 Globally, 382 million people had a diagnosis of diabetes in 2013, and with the increasing prevalence of obesity and adoption of a Western diet, this number is expected to escalate to 592 million by 2035.2
HOW GREAT IS THE CARDIOVASCULAR RISK IN PEOPLE WITH DIABETES?
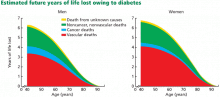
Diabetes mellitus is linked to a twofold increase in the risk of adverse cardiovascular events even after adjusting for risk from hypertension and smoking.3 In early studies, diabetic people with no history of myocardial infarction were shown to have a lifetime risk of infarction similar to that in nondiabetic people who had already had an infarction,4 thus establishing diabetes as a “coronary artery disease equivalent.” Middle-aged men diagnosed with diabetes lose an average of 6 years of life and women lose 7 years compared with those without diabetes, with cardiovascular morbidity contributing to more than half of this reduction in life expectancy (Figure 1).5
Numerous mechanisms have been hypothesized to account for the association between diabetes and cardiovascular risk, including increased inflammation, endothelial and platelet dysfunction, and autonomic dysregulation.6
Can we modify cardiovascular risk in patients with diabetes?
Although fasting blood glucose levels strongly correlate with future cardiovascular risk, whether lowering blood glucose levels with medications will reduce cardiovascular risk has been uncertain.3 Lowering glucose beyond what is current standard practice has not been shown to significantly improve cardiovascular outcomes or mortality rates, and it comes at the price of an increased risk of hypoglycemic events.
No macrovascular benefit from lowering hemoglobin A1c beyond the standard of care
UKPDS.7 Launched in 1977, the United Kingdom Prospective Diabetes Study was designed to investigate whether intensive blood glucose control reduces the risk of macrovascular and microvascular complications in type 2 diabetes. The study randomized nearly 4,000 patients newly diagnosed with diabetes to intensive treatment (with a sulfonylurea or insulin to keep fasting blood glucose levels below 110 mg/dL) or to conventional treatment (with diet alone unless hyperglycemic symptoms or a fasting blood glucose more than 270 mg/dL arose) for 10 years.
Multivariate analysis from the overall study population revealed a direct correlation between hemoglobin A1c levels and adverse cardiovascular events. Higher hemoglobin A1c was associated with markedly more:
- Fatal and nonfatal myocardial infarctions (14% increased risk for every 1% rise in hemoglobin A1c)
- Fatal and nonfatal strokes (12% increased risk per 1% rise in hemoglobin A1c)
- Amputations or deaths from peripheral vascular disease (43% increase per 1% rise)
- Heart failure (16% increase per 1% rise).
While intensive therapy was associated with significant reductions in microvascular events (retinopathy and proteinuria), there was no significant difference in the incidence of major macrovascular events (myocardial infarction or stroke).
The mean hemoglobin A1c level at the end of the study was about 8% in the standard-treatment group and about 7% in the intensive-treatment group. Were these levels low enough to yield a significant risk reduction? Since lower hemoglobin A1c levels are associated with lower risk of myocardial infarction, it seemed reasonable to do further studies with more intensive treatment to further lower hemoglobin A1c goals.
ADVANCE.8 The Action in Diabetes and Vascular Disease trial randomized more than 11,000 participants with type 2 diabetes to either usual care or intensive therapy with a goal of achieving a hemoglobin A1c of 6.5% or less. During 5 years of follow-up, the usual-care group averaged a hemoglobin A1c of 7.3%, compared with 6.5% in the intensive-treatment group.
No significant differences between the two groups were observed in the incidence of major macrovascular events, including myocardial infarction, stroke, or death from any cause. The intensive-treatment group had fewer major microvascular events, with most of the benefit being in the form of a lower incidence of proteinuria, and with no significant effect on retinopathy. Severe hypoglycemia, although uncommon, was more frequent in the intensive-treatment group.
ACCORD.9 The Action to Control Cardiovascular Risk in Diabetes trial went one step further. This trial randomized more than 10,000 patients with type 2 diabetes to receive either intensive therapy (targeting hemoglobin A1c ≤ 6.0%) or standard therapy (hemoglobin A1c 7.0%–7.9%). At 1 year, the mean hemoglobin A1c levels were stable at 6.4% in the intensive-therapy group and 7.5% in the standard-therapy group.
The trial was stopped at 3.5 years because of a higher rate of death in the intensive-therapy group, with a hazard ratio of 1.22, predominantly from an increase in adverse cardiovascular events. The intensive-therapy group also had a significantly higher incidence of hypoglycemia.
VADT.10 The Veterans Affairs Diabetes Trial randomized 1,791 patients with type 2 diabetes who had a suboptimal response to conventional therapy to receive intensive therapy aimed at reducing hemoglobin A1c by 1.5 percentage points or standard therapy. After a follow-up of 5.6 years, median hemoglobin A1c levels were 8.4% in the standard-therapy group and 6.9% in the intensive-therapy group. No differences were found between the two groups in the incidence of major cardiovascular events, death, or microvascular complications, with the exception of a lower rate of progression of albuminuria in the intensive-therapy group. The rates of adverse events, primarily hypoglycemia, were higher in the intensive-therapy group.
Based on these negative trials and concern about potential harm with intensive glucose-lowering strategies, standard guidelines continue to recommend moderate glucose-lowering strategies for patients with diabetes. The guidelines broadly recommend targeting a hemoglobin A1c of 7% or less while advocating a less ambitious goal of lower than 7.5% or 8.0% in older patients who may be prone to hypoglycemia.11