Sudden death in epilepsy, surgery, and seizure outcomes: The interface between heart and brain
ABSTRACT
A significant body of literature suggests that sudden unexpected death in epilepsy (SUDEP) has a cardiac mechanism. Although a correlation has been established between freedom from seizures and reduced SUDEP risk, the exact relationship between seizure outcomes following epilepsy surgery and SUDEP is still being studied. Emerging evidence suggests that seizure outcomes following epilepsy surgery and risk for SUDEP may both be governed by a common underlying biologic process linked to cardiac changes and autonomic dysregulation.
Sudden unexpected death in epilepsy (SUDEP) is most often defined as the sudden, unexpected, nontraumatic, and nondrowning death of patients with epilepsy. The death may be either witnessed or unwitnessed, and may occur with or without evidence of a seizure, excluding documented status epilepticus. In cases of definite SUDEP, postmortem examination does not reveal a structural or toxicologic cause of death.1
The reported incidence of SUDEP ranges from 0.7 to 1.3 cases per 1,000 patient-years in large cohorts of epilepsy patients2,3 and from 3.5 to 9.3 cases per 1,000 patient-years in antiepileptic drug registries, medical device registries, and epilepsy surgery programs.4–6 SUDEP is currently accepted as the most important epilepsy-related mode of death5,7 and is associated with standardized mortality ratios in patients with ongoing seizures as high as 24 to 40 times those of the general population.8 The exact mechanism or mechanisms leading to SUDEP remain unknown,1,5,9,10 and despite the identification of several potential risk factors, elimination of seizures still represents the main intervention correlating with risk reduction.5,11
This review will first discuss evidence for a cardiac mechanism of SUDEP, then review data traditionally used to support freedom from seizures following epilepsy surgery as a method for reducing SUDEP risk, and finally examine recent evidence suggesting that both SUDEP and seizure outcomes following epilepsy surgery are governed by a common pathway with certain cardiac manifestations.
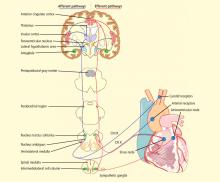
CENTRAL AUTONOMIC NETWORK
EVIDENCE FOR A CARDIAC MECHANISM OF SUDEP
The most significant and broadly discussed cardiac mechanism of SUDEP is cardiac arrhythmia brought about by seizure discharges acting through the autonomic nervous system.5,7,15,16
Clinical evidence
A wide spectrum of cardiac arrhythmias—from ictal asystole to atrial fibrillation to repolarization abnormalities to bundle branch blocks—has been reported during seizures. 14–19 In one study, ictal cardiac arrhythmias occurred in 42% of hospitalized epilepsy patients, the most common being an irregular series of abrupt rate changes near the end of the electroencephalographic (EEG) seizure discharge.17 In another study, R-R interval analysis during the first 10-second period of EEG discharge showed a significant early heart rate increase in 49% of seizures and an early heart rate reduction in 25.5%.18
Certain clinical seizure characteristics have been correlated with the occurrence of ictal electrocardiographic (ECG) abnormalities. One study found that mean seizure duration was longer in patients with ECG abnormalities than in those without such changes.16 Others observed that ictal ECG abnormalities occurred more often and were more severe in generalized tonic-clonic seizures than in complex partial seizures.15,16,19 These same clinical seizure characteristics were correlated with a higher risk of SUDEP,20 which suggests an interrelation among seizure semiology, ECG abnormalities, and SUDEP.
Additionally, direct evidence of seizure-related cardiac changes occurring specifically in SUDEP victims has come from a study that compared EEG and ECG data between 21 SUDEP patients and 43 clinically similar historical controls with refractory partial epilepsy.15 The patients who eventually succumbed to SUDEP had a higher ictal maximal heart rate, a greater increase in heart rate with seizures arising from sleep, and a higher incidence of ictal cardiac repolarization and rhythm abnormalities.15
Experimental evidence
Electrical brain stimulation of the limbic system and insular cortex has repeatedly been shown to provoke heart rate changes, including bradycardia, tachycardia, and asystole.14 Some studies have even suggested a lateralized influence of the insulae on cardiovascular autonomic control, with intraoperative stimulation of the left posterior insula eliciting a cardioinhibitory response and hypotension, and with stimulation of the right anterior insula eliciting tachycardia and hypertension.7 Other studies have suggested that the limbic system has a localization-related influence on cardiovascular responses, with stimulation of the amygdala alone being insufficient to produce the ictal tachycardia so commonly seen in epileptic seizures, which suggests that cortical involvement is essential for the increase in heart rate.21 Such cortical stimulation–induced heart rate changes may explain how massive seizure-related discharges can affect cardiac rhythm during the seizure itself.
There is also, however, evidence of a baseline epilepsy-related autonomic dysfunction. Abnormalities in cardiac uptake of meta-iodobenzylguanidine (MIBG) have been demonstrated interictally in patients with chronic temporal lobe epilepsy relative to controls.22 A recent study specifically showed a pronounced reduction in cardiac MIBG uptake in patients who had ictal asystole compared with other epileptic patients and nonepileptic controls, a finding that suggests a postganglionic cardiac catecholamine disturbance in patients with epilepsy.23 The authors proposed that epilepsy-related impairment of sympathetic cardiac innervation limits adjustment and heart rate modulation, and may thus increase the risk of asystole and, ultimately, SUDEP.23