Should patients stop taking aspirin for primary prevention?
In view of current evidence, we do not recommend routinely using aspirin for primary prevention of cardiovascular disease, even in patients with diabetes mellitus. The decision must be individualized on the basis of the patient’s risks of cardiovascular disease and bleeding, especially the risk of serious bleeding events such as gastrointestinal and intracranial hemorrhage.
For example, patients with a family history of myocardial infarction at an early age and patients who smoke or have multiple cardiovascular risk factors may be most likely to benefit, whereas those with risk factors for gastrointestinal bleeding such as dyspepsia or ulcer would not be good candidates. Of note, current recommendations are mixed and confusing and will need to be reevaluated as new trial data become available.
TRIALS THAT SET THE STAGE FOR CURRENT PRACTICE
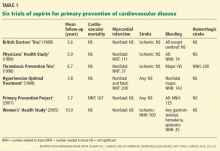
Routine use of aspirin for primary prevention of cardiovascular disease remains controversial.1,2 Aspirin’s safety and efficacy for this indication was studied in six major trials (Table 1).3–8 In the late 1980s, the first two primary prevention trials of aspirin enrolled healthy male physicians who had minimal cardiovascular risk factors3,4:
The British Doctors’ Trial3 observed no significant differences between aspirin (300–500 mg/day) and no aspirin in the rates of the primary end point of cardiovascular death or in the individual secondary end points of nonfatal myocardial infarction, nonfatal stroke, or bleeding.3
The Physicians’ Health Study4 found no differences in the rates of cardiovascular mortality or ischemic stroke between aspirin (325 mg every other day) and placebo. The rate of nonfatal myocardial infarction was significantly lower with aspirin than with placebo, but with a higher risk of bleeding. Relative risks and 95% confidence intervals with aspirin vs placebo:
- Nonfatal myocardial infarction
0.59 (0.47–0.74), P < .00001 - Bleeding
1.32 (1.25–1.40), P < .00001 - Blood transfusions
1.71 (1.09–2.69), P = .02 - Hemorrhagic stroke
2.14 (0.96–4.77), P = .06.
A subgroup analysis revealed that the benefit of aspirin for myocardial infarction in the Physicians’ Health Study was predominantly in those age 50 and older.4 This finding established the common clinical practice of routinely using aspirin for primary prevention in men age 50 and older.1
Later, aspirin for primary prevention was studied in four trials,5–8 three of which enrolled patients at higher cardiovascular risk5–7:
The Thrombosis Prevention Trial5 was conducted in men in the highest quintile of cardiovascular risk. The aspirin dosage was 75 mg/day.
The Hypertension Optimal Treatment6 trial included men and women ages 50 to 80 with hypertension. Aspirin dosage: 75 mg/day.
The Primary Prevention Project7 involved men and women age 50 and older with at least one risk factor for cardiovascular disease.1,5–7 The aspirin dosage was 100 mg/day.
In these trials (Table 1), aspirin significantly lowered the rate of ischemic events compared with placebo or control: nonfatal myocardial infarction in the Thrombosis Prevention Trial; myocardial infarction and major adverse cardiac event (ie, cardiovascular death, myocardial infarction, or stroke) in the Hypertension Optimal Treatment trial; and cardiovascular mortality and major cardiovascular events (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, angina pectoris, transient ischemic attack, peripheral artery disease, or revascularization procedures) in the Primary Prevention Project. However, aspirin’s benefit in each trial was largely offset by a higher rate of various bleeding end points.5–7
The Women’s Health Study
A subgroup analysis of the Hypertension Optimal Treatment trial suggested that sex may influence the efficacy of aspirin—specifically, aspirin did not prevent nonfatal myocardial infarction in women.9 Given the paucity of female participants in the previous primary prevention trials, the Women’s Health Study8 was designed to determine the efficacy and safety of aspirin (100 mg every other day) in women age 45 and older with very few cardiovascular risk factors.8
Aspirin did not significantly reduce the rate of the primary end point of cardiovascular death, myocardial infarction, or stroke, though a significant effect was observed in the subgroup of women age 65 and older. Although overall the Women’s Health Study found no benefit in the rate of myocardial infarction, there was a significant reduction in the rate of ischemic stroke (which needs to be interpreted cautiously in an overall neutral trial) and a nonsignificant increase in the rate of hemorrhagic stroke. As in other trials, rates of bleeding, including gastrointestinal bleeding, were higher with aspirin.
A meta-analysis of six trials of aspirin for primary prevention
In 2009, the Antithrombotic Trialists’ Collaboration10 published a meta-analysis of six trials of aspirin for primary prevention. In this analysis, aspirin did not reduce the rate of cardiovascular death, but it did reduce the yearly risk of:
- Death from coronary heart disease or nonfatal myocardial infarction
(0.28% vs 0.34%, P < .0001) - Nonfatal myocardial infarction
(0.18% vs 0.23%, P < .0001) - Ischemic stroke
(0.11% vs 0.12%, P = .05).10
Despite aspirin’s apparent efficacy, the absolute yearly risk for major extracranial bleeding and hemorrhagic stroke was also significantly increased with aspirin use by 0.3% and 0.1%, respectively. The efficacy of aspirin for preventing all serious vascular events (vascular death, myocardial infarction, or stroke) was similar in men and women.10 The authors concluded that the net benefit of aspirin did not outweigh the increased risks of bleeding.