Selection of chemotherapy for patients with advanced non–small cell lung cancer
ABSTRACTChemotherapy remains the first-line treatment for most patients with stage IV non–small cell lung cancer (NSCLC), but optimal regimens are now defined by histology. Platinum-based regimens with pemetrexed, bevacizumab, or both are reasonable first-line options for patients with nonsquamous NSCLC. The standard treatment for squamous NSCLC remains a platinum doublet with a drug other than pemetrexed. Maintenance therapy is emerging as a treatment strategy for patients who do not progress after four cycles of first-line chemotherapy. In the maintenance setting, pemetrexed and erlotinib significantly prolong overall survival compared with placebo after the completion of first-line chemotherapy.
Despite enthusiasm for the use of molecular testing and molecularly targeted agents in patients with advanced non–small cell lung cancer (NSCLC), most patients are not candidates for upfront treatment with molecular agents. Chemotherapy therefore remains the backbone of treatment for this patient population.
This article presents the best available evidence for selection of chemotherapy for patients with advanced NSCLC and examines the controversy surrounding maintenance therapy.
EVOLUTION OF CHEMOTHERAPY IN NSCLC
The first evidence that chemotherapy produced a significant survival benefit in patients with advanced NSCLC came in 1995 when a meta-analysis showed that platinum-based chemotherapy conferred a 2-month improvement in median survival over best supportive care.1
This finding led to a decade of randomized phase 3 clinical trials that compared different platinum-based regimens. The quintessential trial in this regard was Eastern Cooperative Oncology Group (ECOG) 1594, in which three platinum doublets were compared with cisplatin and paclitaxel on the end point of overall survival (OS) in patients with advanced NSCLC. No significant differences were found among the regimens tested.2
Bevacizumab adds to platinum doublet in nonsquamous NSCLC
With the introduction of bevacizumab, an antibody against vascular endothelial growth factor, knowledge of NSCLC histology became important. In the ECOG 4599 trial, published in 2006, bevacizumab added to platinum doublet chemotherapy in patients with advanced nonsquamous NSCLC significantly improved the response rate, progression-free survival (PFS), and median OS compared with platinum doublet chemotherapy alone.3 This trial was limited to patients with nonsquamous NSCLC because its predecessor trial had revealed an excess of life-threatening pulmonary hemorrhage in association with bevacizumab in patients with squamous cell carcinoma.
Pemetrexed superior to docetaxel in nonsquamous histology
In 2004, Hanna et al4 demonstrated pemetrexed to be noninferior to docetaxel on efficacy outcomes as second-line therapy in advanced NSCLC. Pemetrexed had a significantly better toxicity profile, however, which led to its approval for this indication. Post hoc analyses of this trial suggested a differential effect of pemetrexed based on histology. In the pemetrexed arm, patients with nonsquamous histology appeared to have superior survival compared with patients who had squamous histology, whereas in the docetaxel arm, histology did not affect outcome.5 Further, the nonsquamous histologic subgroup had superior OS with pemetrexed compared with docetaxel.6
Pemetrexed and gemcitabine perform differently based on histology
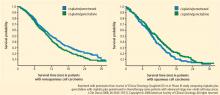
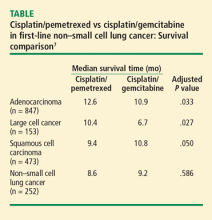
A phase 3 trial with a noninferiority design compared cisplatin/pemetrexed with cisplatin/gemcitabine as first-line therapy on OS. The noninferiority criteria were met, with no difference in median OS between the two groups (median OS: 10.3 months in both arms).7
Based on this evidence, NSCLC is no longer an adequate pathologic diagnosis. Pathologists must differentiate squamous from nonsquamous histology to take full advantage of the safety of angiogenesis inhibitors and the efficacy of pemetrexed.