New Therapeutic Frontiers in the Treatment of Eosinophilic Esophagitis





Evan S. Dellon, MD, MPH
Professor Medicine;
Director of the Center for Esophageal Diseases and Swallowing,
Department of Medicine, Division of Gastroenterology
University of North Carolina School of Medicine
Chapel Hill, North Carolina
Evan S. Dellon, MD, MPH, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Abbvie; Adare/Ellodi; Akesobio; Alfasigma; ALK; Allakos; Amgen; Apollo; Aqilion; Arena/Pfizer; Aslan; AstraZeneca; Avir; Biocryst; Bryn; Calypso; Celgene/Receptos/BMS; Celldex; EsoCap; Eupraxia; Dr. Falk Pharma; Ferring; GI Reviewers; GSK; Holoclara; Invea; Knightpoint; LucidDx; Morphic; Nexstone Immunology/Uniquity; Nutricia; Parexel/Calyx; Phathom; Regeneron; Revolo; Robarts/Alimentiv; Sanofi; Shire/Takeda; Target RWE; Upstream Bio Received research grant from: Adare/Ellodi; Allakos; Arena/Pfizer; AstraZeneca; Celldex; Eupraxia; Ferring; GSK; Meritage; Miraca; Nutricia; Celgene/Receptos/BMS; Regeneron; Revolo; Sanofi; Shire/Takeda Received educational grant from: Allakos; Aqilion; Holoclara; Invea





Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease characterized by symptoms of esophageal dysfunction and dense eosinophilic infiltration. The prevalence of EoE continues to increase in the United States, with genetic, environmental, and microbiome factors contributing to its rise.1 This condition manifests as dysphagia for solid food in adults and adolescents and can lead to esophageal remodeling if untreated; symptoms are non-specific in children. Diagnosis of EoE is per International Consensus Criteria.2 Management options include proton pump inhibitors, elimination diets, topical steroids, and biologics.1
Recent advances in treatment include the US FDA approval of the swallowed topical steroid budesonide oral suspension and of a monoclonal antibody targeting interleukins (IL)-4/IL-13 (dupilumab).3 The positioning of biologics continues to evolve but, as for other atopic conditions, they are mostly used as “step-up” treatment for more difficult-to-treat patients with EoE. The Index of Severity of Eosinophilic Esophagitis (I-SEE), developed in 2022, has been shown to be a promising clinical tool for assessing and following EoE severity that may ultimately help to better manage treatment modalities.4,5 After prescribing treatment, careful assessment of symptomatic, endoscopic, and histologic outcomes is needed to determine response.3 In addition, understanding of various inflammatory mechanisms has led to the ongoing development and evaluation of new biological drugs targeting the Th2 axis and fibrosis.1,3 More studies are needed to determine the effects of these emerging therapies as well as the long-term outcomes of existing treatments for patients with EoE.
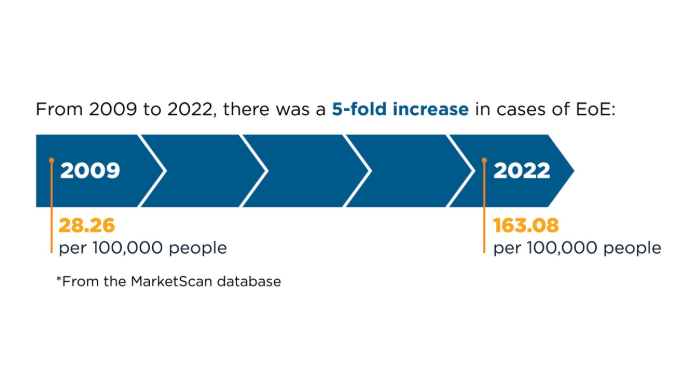 Prevalence and Cost of EoE in the US6,7
Prevalence and Cost of EoE in the US6,7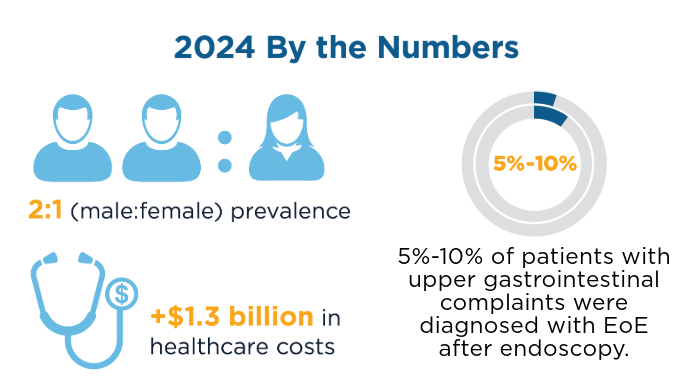 Prevalence and Cost of EoE in the US6,7
Prevalence and Cost of EoE in the US6,7
There has been a marked increase in EoE prevalence over the past 10+ years, in all age groups and sexes. Because the prevalence of EoE is particularly high in patients presenting with symptoms of dysphagia or food impaction, it is important to consider the diagnosis and perform esophageal biopsies during endoscopy for all patients with dysphagia regardless of the appearance and whenever EoE is on the differential diagnosis.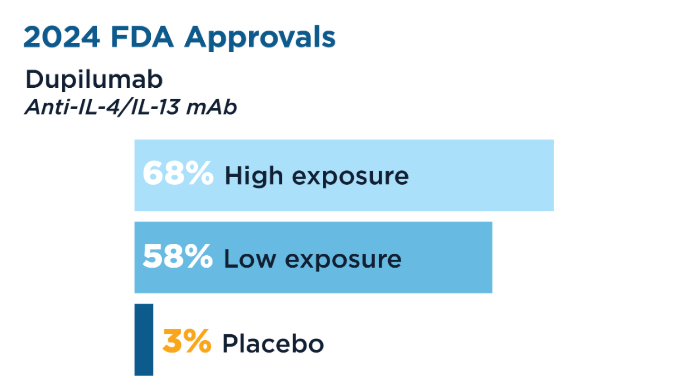 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14
The EoE KIDS trial demonstrated histologic remission in children aged 1 to 11 years who did not respond to proton pump inhibitors (PPIs), leading to the January 2024 expanded FDA approval in patients aged ≥ 1 year and weight ≥ 15 kg. The original 2022 FDA approval in patients aged 12+ and weighing 40+ kg was based on the LIBERTY EoE TREET study.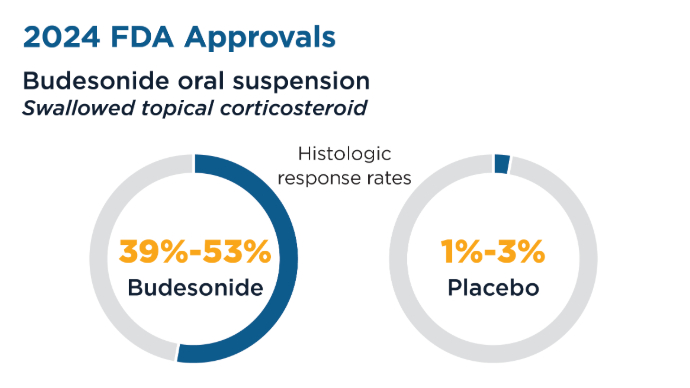 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14
Two 12-week studies demonstrated significantly improved histologic remission and patient reported dysphagia in patients aged 11 years, leading to FDA approval in February 2024.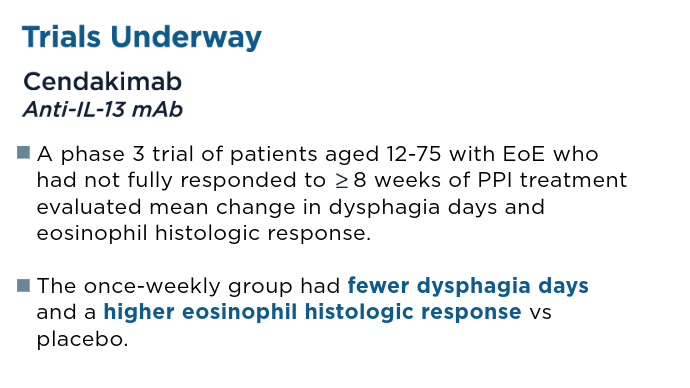 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14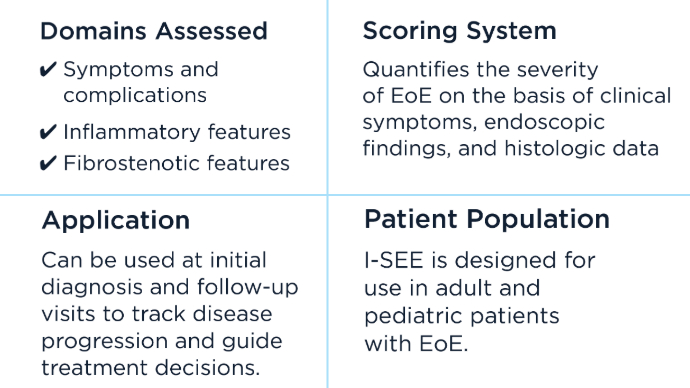 The Index of Severity of Eosinophilic Esophagitis, I-SEE Tool4,5
The Index of Severity of Eosinophilic Esophagitis, I-SEE Tool4,5
The I-SEE tool, which is completed by the clinician, uses a standardized scoring system designed to assess and track the severity of EoE by evaluating various domains. I-SEE helps clinicians quantify disease activity and monitor progression over time, guiding treatment decisions and improving disease management. Prevalence and Cost of EoE in the US6,7
Prevalence and Cost of EoE in the US6,7 Prevalence and Cost of EoE in the US6,7
Prevalence and Cost of EoE in the US6,7
There has been a marked increase in EoE prevalence over the past 10+ years, in all age groups and sexes. Because the prevalence of EoE is particularly high in patients presenting with symptoms of dysphagia or food impaction, it is important to consider the diagnosis and perform esophageal biopsies during endoscopy for all patients with dysphagia regardless of the appearance and whenever EoE is on the differential diagnosis. Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14
The EoE KIDS trial demonstrated histologic remission in children aged 1 to 11 years who did not respond to proton pump inhibitors (PPIs), leading to the January 2024 expanded FDA approval in patients aged ≥ 1 year and weight ≥ 15 kg. The original 2022 FDA approval in patients aged 12+ and weighing 40+ kg was based on the LIBERTY EoE TREET study. Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14
Two 12-week studies demonstrated significantly improved histologic remission and patient reported dysphagia in patients aged 11 years, leading to FDA approval in February 2024. Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 The Index of Severity of Eosinophilic Esophagitis, I-SEE Tool4,5
The Index of Severity of Eosinophilic Esophagitis, I-SEE Tool4,5
The I-SEE tool, which is completed by the clinician, uses a standardized scoring system designed to assess and track the severity of EoE by evaluating various domains. I-SEE helps clinicians quantify disease activity and monitor progression over time, guiding treatment decisions and improving disease management. Prevalence and Cost of EoE in the US6,7
Prevalence and Cost of EoE in the US6,7 Prevalence and Cost of EoE in the US6,7
Prevalence and Cost of EoE in the US6,7
There has been a marked increase in EoE prevalence over the past 10+ years, in all age groups and sexes. Because the prevalence of EoE is particularly high in patients presenting with symptoms of dysphagia or food impaction, it is important to consider the diagnosis and perform esophageal biopsies during endoscopy for all patients with dysphagia regardless of the appearance and whenever EoE is on the differential diagnosis. Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14
The EoE KIDS trial demonstrated histologic remission in children aged 1 to 11 years who did not respond to proton pump inhibitors (PPIs), leading to the January 2024 expanded FDA approval in patients aged ≥ 1 year and weight ≥ 15 kg. The original 2022 FDA approval in patients aged 12+ and weighing 40+ kg was based on the LIBERTY EoE TREET study. Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14
Two 12-week studies demonstrated significantly improved histologic remission and patient reported dysphagia in patients aged 11 years, leading to FDA approval in February 2024. Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 Recently Approved and Emerging EoE Treatments8-14
Recently Approved and Emerging EoE Treatments8-14 The Index of Severity of Eosinophilic Esophagitis, I-SEE Tool4,5
The Index of Severity of Eosinophilic Esophagitis, I-SEE Tool4,5
The I-SEE tool, which is completed by the clinician, uses a standardized scoring system designed to assess and track the severity of EoE by evaluating various domains. I-SEE helps clinicians quantify disease activity and monitor progression over time, guiding treatment decisions and improving disease management.
