Nephrogenic systemic fibrosis and its association with gadolinium exposure during MRI
ABSTRACTNephrogenic systemic fibrosis (NSF) is a newly recognized systemic disorder characterized by widespread tissue fibrosis in patients with impaired renal function. Recent reports suggest that NSF is associated with exposure to gadolinium-based contrast agents used in magnetic resonance imaging. NSF can be very debilitating and can lead to serious complications and death. Health care providers should exercise caution when considering the use of gadolinium-based imaging studies in patients with renal dysfunction.
KEY POINTS
- NSF seems to arise in roughly 3% of patients with renal insufficiency who receive gadolinium, although the data are somewhat sketchy and the true incidence might be higher if the NSF is specifically looked for.
- Manufacturers of all available gadolinium contrast agents now must include a boxed warning about the risk of NSF in patients with acute or chronic severe renal insufficiency (glomerular filtration rate < 30 mL/minute/1.73 m2) and in patients with acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period.
- As yet, we have no effective treatment for NSF. If the patient is already on hemodialysis, it may be reasonable to perform hemodialysis immediately after exposure to gadolinium and again the next day.
The use of gadolinium as a contrast agent in magnetic resonance imaging (MRI) in patients with impaired kidney function has come under scrutiny because of recent reports of a potential association between its use and nephrogenic systemic fibrosis (NSF).
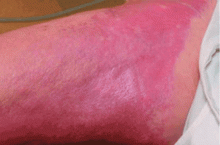
This entity was first identified in the United States in 1997. Cowper et al1 in 2000 described 15 hemodialysis patients who developed thickening and hardening of the skin with brawny hyperpigmentation, papules, and subcutaneous nodules on the extremities.
This “new disease” was initially called “nephrogenic fibrosing dermopathy,” as it was exclusively seen in patients with renal impairment and was thought to affect only the skin and subcutaneous tissue. With growing evidence of the extent and pathogenicity of the fibrosis in visceral organs, the nomenclature was changed to NSF, to better reflect the systemic nature of the disease.
PRESENTATION: MILD TO DEVASTATING
NSF has thus far been reported only in patients with renal impairment, most of whom were dialysis-dependent. It does not seem to be more common in one sex or the other, in any age range, or in any ethnic group. It can range in severity from mild to a devastating scleroderma-like systemic fibrosing disorder.
The heart, lungs, skeletal muscle, and diaphragm can also be involved, sometimes leading to serious complications and death.4–6
The disease is usually progressive and unremitting. Mendoza et al,7 in a review of 12 cases of NSF, reported that the disease had a progressive course in 6 patients, of whom 3 died within 2 years and 3 were ultimately confined to a wheelchair. More severe findings and rapid progression of the skin disease are associated with a poor prognosis.
Todd et al8 prospectively examined 186 dialysis patients to look for possible NSF. Of those with skin changes consistent with NSF, 48% died within 2 years, compared with 20% of those without these skin changes. Cardiovascular causes accounted for 58% of the deaths in patients with cutaneous changes of NSF and for 48% of the deaths in patients without these changes. Most of the excess deaths occurred within 6 months after the skin examination, suggesting an increased risk for early death in patients with skin changes suggestive of NSF.
DIAGNOSIS OF NSF IS CLINICAL
At presentation, NSF is frequently misdiagnosed and treated as cellulitis or edema. However, now that subspecialists—especially dermatologists, rheumatologists, and nephrologists—are becoming more aware of it, the correct diagnosis is being made earlier.
NSF should be suspected in any patient with underlying renal dysfunction—especially if on dialysis and if he or she has received a gadolinium contrast agent during MRI—who develops scleroderma-like cutaneous lesions affecting the distal extremities. Because most health care providers are still unfamiliar with this emerging disease, patients with renal impairment and suspected NSF should be referred to a rheumatologist or dermatologist to confirm the diagnosis, which is mainly entertained on a clinical basis. There is no laboratory biomarker for NSF.
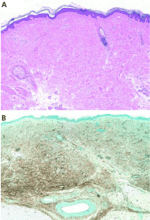
A deep incisional skin biopsy may aid in the diagnosis. Due to the regional distribution of the disease, sampling error may occur, and repeat biopsy is warranted if the initial biopsy is nondiagnostic but the clinical picture suggests NSF.
Histopathologic examination typically shows lesions containing proliferation of dermal spindle cells, thick collagen bundles with surrounding clefts, and a variable amount of mucin and elastic fibers.2 A characteristic and almost pathognomonic staining profile is the immunohistochemical identification of CD34 reactivity in the fibroblast-like cells (Figure 3). Cells expressing CD34 are normally found in the umbilical cord, the bone marrow (as pluripotential hematopoietic stem cells), and in the vascular endothelium. How they come to be in the skin is still speculative, but their presence suggests that circulating fibrocytes migrate from the bone marrow and deposit in the skin and other organs.9,10
Pulmonary function testing can be done to rule out lung involvement and transthoracic two-dimensional echocardiography can be done to rule out possible cardiomyopathy if these conditions are suggested by examination at the time of diagnosis.7 Muscle biopsy is not necessary to determine the extent of systemic involvement, since the findings do not necessarily correlate with other systemic involvement.