Long-term management of liver transplant recipients: A review for the internist
ABSTRACTOutcomes and long-term survival rates after liver transplantation have significantly improved over the last 5 decades, but the improved longevity is accompanied by long-term complications such as metabolic syndrome, obesity, cardiovascular disease, cancer, and bone disease. After the first year, the primary care physician assumes a greater role and provides most of the patient’s care. This review provides a source for primary care physicians in managing the long-term medical complications seen in these patients.
KEY POINTS
- Tacrolimus and cyclosporine are the most commonly used immunosuppressive agents in liver transplant recipients. Adverse effects include hypertension, hypercholesterolemia, diabetes (more common with tacrolimus), renal insufficiency, and osteoporosis.
- Hypertension affects 40% to 85% of liver transplant patients. Dihydropyridine calcium channel blockers (eg, amlodipine, nifedipine) are the first-line agents.
- Cardiovascular disease is the third most common cause of death after liver transplantation. Modifying risk factors is essential.
- Skin cancers account for 40% of all cancers after liver transplantation. Intensive screening is required and has been proven to lower the risk of death.
Since 1963, when Starzl et al performed the first successful liver transplantation,1 outcomes of this life-saving procedure have continued to improve. Long-term survival rates have increased markedly: the current 5-year rate is 73.8% and the 10-year rate is 60%.2
This success means that internists will be caring for a greater number of liver transplant recipients and managing their long-term problems, such as hypertension, diabetes mellitus, dyslipidemia, obesity, metabolic syndrome, cardiovascular disease, renal insufficiency, osteoporosis, cancer, and gout.
This review will discuss these complications, focusing on the role the primary care physician assumes beyond the first year after transplantation.
ROLE OF THE PRIMARY CARE PHYSICIAN
Hepatologists, primary care physicians, and surgeons share the care of transplant recipients. The first several weeks after transplantation require close follow-up by the hepatologist and transplantation team, with particular attention paid to the patient’s overall health and well-being, medication compliance, and biochemical and immunosuppression monitoring.
After the first year, the primary care physician assumes a greater role, becoming the main provider of the patient’s care.3,4 Good communication between the transplant center and the primary care physician should lead to a smooth transition.4 Although the hepatologist continues to manage immunosuppressive drugs, allograft rejections, and biliary complications, the primary care physician manages most of the long-term complications and thus needs to be aware of the common ones and feel comfortable managing them. Aims during visits are to screen for and detect common complications and manage them appropriately, in addition to performing annual physical examinations and routine health care. A reasonable interval for liver transplant recipients to visit their primary care physician is every 6 months.
IMMUNOSUPPRESSANT MEDICATIONS
Multiple agents are used for immunosuppression after liver transplantation:
- Calcineurin inhibitors (cyclosporine and tacrolimus)
- Antimetabolites (mycophenolate mofetil, azathioprine, and mycophenolate sodium)
- Mammalian target of rapamycin (mTOR) inhibitors (sirolimus and everolimus)
- Corticosteroids.
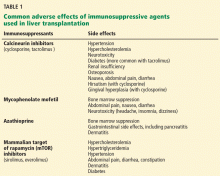
Table 1 lists their common side effects.
Most centers use a combination of two to four immunosuppressants as induction therapy in the immediate posttransplant period, then taper the doses and eliminate all but a calcineurin inhibitor and an antimetabolite. For example, some start with a combination of tacrolimus, mycophenolate mofetil, and a corticosteroid. The choice in the immediate posttransplant period is frequently made by the transplant center in cooperation with the hepatologist. By the time primary care physicians see these patients, they usually are on a calcineurin inhibitor alone or a calcineurin inhibitor plus mycophenolate mofetil.
Calcineurin inhibitors
Cyclosporine is metabolized by the cytochrome CYP3A4 pathway. With an average half-life of 15 hours, it is given orally, usually every 12 hours.
The dosage is adjusted according to the trough level. Higher levels are needed in the initial posttransplant period to prevent graft rejection, whereas lower levels are preferred later to decrease the occurrence and severity of adverse effects. Typical long-term trough levels are 50 to 100 ng/mL. Levels should be checked more often if an acute illness develops or the patient starts taking a potentially interfering drug.
Of importance: the dosage should be based on trough levels and not on random levels. Levels are often falsely high if blood samples are not drawn at the trough level. Repeating the measurement and making sure the sample is drawn at the trough level, ie, 12 hours after the last dose, is advised in this condition.
Cyclosporine causes widespread vasoconstriction resulting in decreased renal blood flow and systemic hypertension, often within a few days of starting it. Other important adverse effects include renal insufficiency, dyslipidemia, neurotoxicity (headache, tremor, seizure), and diabetes.
Tacrolimus is superior to cyclosporine in terms of survival, graft loss, acute rejection, and steroid-resistant rejection in the first year.5 Currently, it is the agent used most often for maintenance immunosuppression after liver transplantation.
Like cyclosporine, tacrolimus is metabolized in the liver by CYP3A4. Satisfactory trough levels after 1 year are 4 to 6 ng/mL.
The adverse effects of tacrolimus are similar to those of cyclosporine, but diabetes mellitus is more common with tacrolimus. Bone marrow suppression may occur more often with tacrolimus as well.
Antimetabolites
Antimetabolites are generally not potent enough to be used alone.
Mycophenolate mofetil causes adverse effects that include bone marrow suppression and gastrointestinal symptoms such as gastritis, diarrhea, and abdominal pain.
Azathioprine, infrequently used in transplantation in the United States, is nevertheless sometimes substituted for mycophenolate mofetil in pregnant women, as it seems safer for use in pregnancy.
Serum levels of azathioprine and mycophenolate mofetil are not routinely monitored.
mTOR inhibitors
Sirolimus and everolimus are mTOR inhibitors, inhibiting proliferation of lymphocytes.6,7
Unlike calcineurin inhibitors, mTOR inhibitors are not associated with nephrotoxicity, neurotoxicity, renal dysfunction, hypertension, or diabetes. Sirolimus is considered an alternative to calcineurin inhibitors or, in some instances, used as add-on-therapy to lower the dose of the calcineurin inhibitor.
However, sirolimus carries a potential risk of hepatic artery thrombosis, a life-threatening complication.8 This has led the US Food and Drug Administration (FDA) to require sirolimus to carry a black-box warning, and most transplant centers avoid using it in the first 30 days after transplantation.
Dyslipidemia is perhaps the most common adverse effect of sirolimus. Others include dose-related cytopenia and wound dehiscence.9
Everolimus has yet to be established for use in liver transplantation, although safety trials have been published.10,11 The FDA currently recommends against using it in the first 30 days after liver transplantation.
Both sirolimus and everolimus are metabolized by CYP3A4, which is the same metabolic pathway used by cyclosporine and tacrolimus. Hence, drugs that inhibit CYP3A4 may significantly impair clearance of both sirolimus and everolimus.