Resuming anticoagulation after hemorrhage: A practical approach
ABSTRACTMost patients who suffer a hemorrhage while on long-term anticoagulant therapy continue to be at risk of thrombosis. Physicians often need to reconsider the need for anticoagulation in view of the risk of recurrent bleeding, and when anticoagulation needs to be resumed, they must also consider the timing and strategy. Since there are no evidence-based guidelines for these situations, the authors of this paper offer a practical framework for individualizing the resumption of anticoagulation after hemorrhage.
KEY POINTS
- Not all patients on anticoagulation at the time of a bleeding event have a strong indication to continue anticoagulation afterward.
- Important considerations when deciding whether to resume anticoagulation after hemorrhage are whether the source of bleeding has been found and controlled and, if the patient is receiving warfarin, whether he or she can be expected to maintain the target international normalized ratio.
- The newer oral anticoagulants, including factor Xa inhibitors and direct thrombin inhibitors, lack antidotes or reversal agents, and their risk of causing bleeding compared with warfarin varies by site of bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
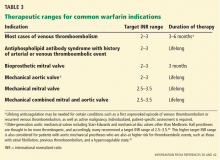
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.