Don't forget non-Alzheimer dementias
ABSTRACTDementia is commonly encountered in the elderly, with prevalence increasing with age. Although Alzheimer disease is the most recognized form of dementia, other types have distinct clinical features and are often overlooked. Proper identification aids patients, caregivers, and physicians in planning and management.
KEY POINTS
- Vascular dementia presents as a sudden, stepwise progression of cognitive deficits.
- Lewy body dementia often involves prominent visual hallucinations.
- Progressive supranuclear palsy starts with gait and balance problems caused by downward-gaze palsy.
- Many neurodegenerative conditions involve parkinsonism, but unlike Parkinson disease, they do not tend to respond well to levodopa, and dementia develops early.
- Corticobasal degeneration involves markedly asymmetric parkinsonism.
- Frontotemporal dementia involves dramatic behavior changes, including inappropriate impulsivity and complete apathy.
- Patients with rapidly progressive dementia should be evaluated for a treatable condition such as antibody-mediated encephalitis.
DEMENTIA WITH LEWY BODIES
Dementia with Lewy bodies, the next most common neurodegenerative dementia in the elderly, is characterized by progressive loss of cognitive function, prominent visual hallucinations, and parkinsonism (Table 1).6 Disease progression usually occurs over years but can be more rapid than in Alzheimer disease.
Alpha-synucleinopathy results in dysfunction of synaptic vesicles in presynaptic terminals. Lewy bodies may be diffusely spread in cortical and subcortical areas (appearing as spherical masses).
Visual hallucinations are typical
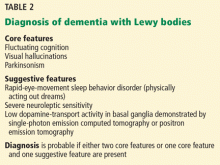
The McKeith criteria16 are the gold standard for diagnosing probable Lewy body dementia, based on clinical and imaging features (Table 2).
Visual hallucinations are usually well formed and detailed. They may initially be pleasant (eg, seeing children and little people) but may evolve to be accompanied by persecutory delusions.
Parkinsonism develops with or after dementia with Lewy bodies
Dementia with Lewy bodies and Parkinson disease dementia share many clinical and pathologic features; Parkinson dementia also is associated with cortical Lewy bodies.
Parkinsonian features include bradykinesia, masked facies, and rigidity. Resting tremor is less common.
The third report of the Dementia With Lewy Bodies Consortium recommends that the condition be diagnosed if dementia occurs before or concurrently with parkinsonism, and dementia with Parkinson disease should be diagnosed if dementia occurs in the context of well-established Parkinson disease.16 The development of dementia within 12 months of extrapyramidal signs suggests dementia with Lewy bodies.
Cognitive deficits fluctuate
Cognitive impairment in Lewy body dementia is characterized by progressive dementia with fluctuations in cognitive performance. Family members or caregivers may report that the patient can carry on a conversation one day and the next day be confused and inattentive. Compared with those with Alzheimer dementia, patients with Lewy body dementia have better delayed recall but more problems with executive functioning (planning) and visuospatial skills (following an unfamiliar route, copying a figure).
Specialized imaging provides clues
Dementia with Lewy bodies is associated with diffuse brain atrophy, with no established characteristic pattern on structural neuroimaging with computed tomography (CT) or magnetic resonance imaging (MRI).17 The contrast agent ioflupane iodine-123 injection (DaTscan) used with single-photon emission CT (SPECT) detects dopamine transporters, which are reduced in parkinsonian syndromes. The scan can also help differentiate between Alzheimer dementia and Lewy body dementia by detecting the loss of functional dopaminergic terminals in the striatum in Lewy body dementia. Alpha-synuclein imaging may become another useful diagnostic tool in the future.
Alzheimer medications may help in dementia with Lewy bodies
Medications with anticholinergic effects and dopamine agonists should be discontinued because of possible effects on cognitive function and parkinsonism. In one clinical trial,18 rivastigmine (Exelon) was found to help cognitive functioning as well as reduce psychotic symptoms in dementia with Lewy bodies, although a recent Cochrane review could not support the evidence for use of all cholinesterase inhibitors in Lewy body dementia.19 In another trial,20 memantine (Namenda) was found to improve global clinical status and behavioral symptoms of Lewy body dementia.
Treating hallucinations of dementia with Lewy bodies
Patients with dementia with Lewy bodies are extremely sensitive to the extrapyramidal side effects of neuroleptic drugs. Some evidence indicates that the atypical antipsychotic drug quetiapine (Seroquel) helps with prominent and disturbing psychotic features and is less likely to worsen parkinsonism than other antipsychotics.21 The best evidence is for clozapine (Clozaril) as a treatment for hallucinations in Parkinson dementia, but the possible side effect of agranulocytosis limits its clinical use. Other atypical antipsychotics such as risperidone (Risperdal) and olanzapine (Zyprexa) are not recommended.22
PROGRESSIVE SUPRANUCLEAR PALSY
Progressive supranuclear palsy is a sporadic atypical parkinsonian disorder with onset between age 50 and 70. Familial cases are infrequent.
Progressive supranuclear palsy presents as early postural instability, vertical supranuclear gaze palsy, and axial muscle rigidity in the first few years. Disease progression is gradual: one study of 50 patients found that the median time from onset to the first key motor impairment (unintelligible speech, no independent walking, inability to stand unassisted, wheelchair-bound, or recommendation for feeding tube placement) was 4 years.23
Histologically, progressive supranuclear palsy is characterized by accumulation of tau protein aggregates in the basal ganglia, brainstem, and cerebral cortex. The degenerative process involves dopaminergic, cholinergic, and gamma-aminobutyric acid (GABA)-ergic neurons.24
Gait and balance problems predominate early in progressive supranuclear palsy
The most commonly used diagnostic criteria are from the National Institute of Neurological Disorders and Stroke. The diagnosis of probable progressive supranuclear palsy requires vertical gaze palsy and falls or the tendency to fall within the first year of disease onset and exclusion of other causes.
The earliest symptom is usually gait and balance impairment.25 Falls (usually backward) and postural instability occur during the first year in 58% of patients.26 Instead of turning en bloc as in Parkinson disease, patients with progressive supranuclear palsy tend to pivot quickly. Patients may also have a coarse groaning voice and moaning. Insomnia has been reported, but rapid-eye-movement sleep behavior disorders are infrequent (unlike in Parkinson disease, multiple system atrophy, and Lewy body dementia).27
Apathy and extreme mood swings
Cognitive impairment is seen in 50% of patients in the early stage of progressive supranuclear palsy. It mostly involves the frontal lobe, including frontal behavioral disturbances (eg, apathy in 91% of patients26 or pseudobulbar affect and extreme emotional lability) and deficits in abstract thoughts or verbal fluency (to test this, patients are asked to say as many words as possible from a category in a given time). Ideomotor apraxia (inability to correctly imitate hand gestures and voluntarily pantomime tool use, such as pretending to brush hair) is rare, despite corticobasal degeneration.28
Vertical gaze palsy
The hallmark of progressive supranuclear palsy is vertical gaze palsy. Initially, this involves slowing of vertical saccades, followed by diminished vertical gaze and more characteristic downward gaze palsy. These findings may develop over 3 to 4 years. Vertical gaze palsy leads to spilling food and tripping while walking.
The gaze abnormality combined with rare blinking and facial dystonia form the classic facial expression of astonishment called “leonine facies.” The face is stiff and deeply furrowed, with a look of surprise.
Axial (especially neck) rigidity is more prominent than limb rigidity. Retrocollis (the head is drawn back) occurs in less than 25% of patients. Parkinsonian features such as bradykinesia affect nearly half of patients by the time of diagnosis.
Instead of the classic symptoms of progressive supranuclear palsy, about one-third of patients present with progressive supranuclear palsy-parkinsonism, which involves asymmetric parkinsonism that initially responds to levodopa.29
MRI shows ‘hummingbird sign’
Brain MRI shows atrophy of the brainstem, particularly the midbrain. Thinning of the superior part of the midbrain and dilation of the third ventricle (“hummingbird sign” on sagittal sections or “morning glory flower” on axial sections) support a diagnosis of progressive supranuclear palsy and differentiate it from Parkinson disease and other atypical parkinsonian disorders.30,31
Levodopa ineffective for supranuclear palsy
There is no treatment to slow progressive supranuclear palsy. Even in high doses, levodopa rarely alleviates parkinsonian features in a clinically meaningful way.26 Successful experimental biologic therapies have been studied in animal models.32 Davunetide is thought to help with neuronal integrity and cell survival through the stabilization of microtubules in preclinical studies, but it has not been used in clinical practice.33