Managing risks of TNF inhibitors: An update for the internist
ABSTRACTTumor necrosis factor (TNF) inhibitors have many beneficial effects, but they also pose infrequent but significant risks, including serious infection and malignancy. These risks can be minimized by judicious patient selection, appropriate screening, careful monitoring during treatment, and close communication between primary care physicians and subspecialists.
KEY POINTS
- Over the past 10 years, TNF inhibitors have substantially altered the management of autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease.
- Safety concerns include risks of infection, reactivation of latent infection (eg, fungal infection, granulomatous infection), malignancy, and autoimmune and neurologic effects.
- Before treating, take a complete history, including exposure to latent infections and geographic considerations, and bring patients’ immunizations up to date.
- Regular clinical and laboratory monitoring during treatment helps optimize therapy and minimize the risk of adverse effects.
- Physicians must be aware of atypical presentations of infection and understand how their treatment may differ in patients on biologic therapy.
Perform an examination
A full physical examination with special attention to skin rashes should be performed. This may serve as a baseline to assist early detection of new rashes associated with anti-TNF therapy.
A baseline complete blood cell count and complete metabolic panel should be routinely obtained before starting therapy (and thereafter at the discretion of the physician). In conjunction with follow-up tests, they can help detect an unexpected decrease in white blood cell count or abnormal results on the liver panel.16 These baseline and follow-up tests are generally performed by the subspecialist, and the results are shared with the primary care physician.
Table 2 summarizes key information to be sought before starting a patient on a TNF inhibitor.
ADVERSE EFFECTS OF ANTI-TNF DRUGS
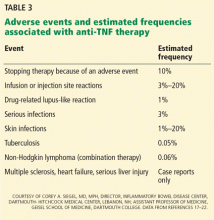
Infusion reactions, infections, cardiac arrhythmias, demyelinating disorders, skin infections, and malignancies have been reported with anti-TNF therapy. The relative frequencies of these adverse events are summarized in Table 3.17–22
NONINFECTIOUS COMPLICATIONS OF TNF INHIBITORS
Injection site reactions
When anti-TNF agents are given subcutaneously, injection site reactions are common (occurring in up to 40% of patients) and are considered minor.11 Reactions, including significant pain, typically occur within the first few months of therapy. They can last 2 to 5 days but rarely warrant stopping therapy. Treatment with ice and an antihistamine is almost always sufficient to control symptoms.
Infusion reactions with infliximab
Infliximab can cause both acute and delayed infusion reactions. Acute reactions can occur up to 24 hours after infusion but usually appear within 10 minutes of administration and are handled by the infusion suite staff. They range from the severe immunoglobulin E-mediated type I reaction, manifesting with hypotension, bronchospasm, and urticaria, to the milder anaphylactoid-type reaction, which constitutes the majority.23–25
While most primary care physicians will not encounter an acute reaction, family doctors and emergency room physicians may encounter delayed reactions, which can develop 1 to 14 days after infusion. These reactions usually resemble serum sickness and present with joint pain, fatigue, myalgia, and fever. But, unlike classic serum sickness, these reactions are generally not associated with a rash.
With nonspecific symptoms, the diagnosis may be easy to overlook. However, establishing this diagnosis is important because repeat therapy may result in a more severe reaction upon reexposure to the drug.
Once diagnosed, these reactions can be treated symptomatically with a combination of acetaminophen and diphenhydramine after discussion between the primary care physician and subspecialist.23,24
Autoimmune syndromes
Several studies have reported a small percentage of patients treated with anti-TNF agents who develop paradoxical autoimmune conditions. These range from asymptomatic immunologic alterations, including the formation of antinuclear antibodies and antibodies to double-stranded DNA, to life-threatening systemic autoimmune diseases.26,27
Autoimmune diseases associated with anti-TNF treatment include a lupus-like syndrome, vasculitides, and psoriatic skin lesions. These syndromes warrant stopping the inciting drug and, on occasion, giving corticosteroids. Most cases arise between 1 month and 1 year of starting treatment, and almost 75% resolve completely after the anti-TNF therapy is stopped.26
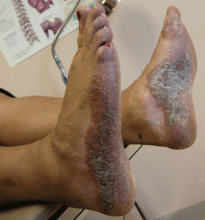
Interestingly, anti-TNF agents are approved for treating psoriasis and psoriatic arthritis, but psoriasis has paradoxically developed in patients being treated with these drugs for other autoimmune diseases. The FDA has reviewed 69 cases of new-onset psoriasis with anti-TNF therapy, including 17 pustular and 15 palmoplantar cases. The 12 most severe cases resulted in hospitalization, and symptoms resolved in most after treatment cessation.28
Fiorino et al29 counted 18 reported cases of psoriasis induced by anti-TNF therapy in patients with inflammatory bowel disease and concluded that it is rare. Harrison et al30 reported similar findings in patients with rheumatoid arthritis, with an increased incidence rate of 1.04 per 1,000 person-years. New-onset psoriasis was most common in patients treated with adalimumab. An example of the rash is seen in Figure 1.
CARDIOVASCULAR SIDE EFFECTS
Cardiovascular side effects of anti-TNF agents range from nonspecific and asymptomatic arrhythmias to worsening of heart failure.
Circulating levels of TNF are increased in patients with heart failure, and studies have evaluated the effects of TNF inhibition with infliximab on cardiac function and overall survival.31,32 The combined risk of death from any cause or hospitalization from heart failure was significantly higher in the infliximab groups, and the effects persisted for up to 5 months after stopping therapy.
Other studies22,33 have evaluated the effects of infliximab and etanercept on cardiac function and overall survival. Results showed possible exacerbation of heart failure with etanercept and increased risk of death with infliximab in patients with New York Heart Association (NYHA) class III or IV heart failure and left ventricular ejection fractions less than 35%.
Case reports have also described patients with worsening or new-onset heart failure on TNF inhibitors, including patients younger than 50 years and without identifiable cardiovascular risk factors.
Data analyses31,34,35 from large clinical registries have reported no significant increase in heart failure attributable to TNF inhibitors. However, we have concerns about the methodology of these analyses.
Currently, anti-TNF therapy is contraindicated in patients with NYHA class III or IV heart failure. Data are inconclusive for patients with class I or II heart failure. Baseline echocardiography and cardiology consultation can be considered, with close monitoring and avoidance of high doses of TNF inhibitors. If heart failure develops in a patient on anti-TNF therapy, the drug should be discontinued and the patient should be evaluated further.36