Incidental ovarian cysts: When to reassure, when to reassess, when to refer
ABSTRACTOvarian cysts are commonly found on imaging done for other reasons. Proper triage will decrease unnecessary procedures and worry while obtaining the best survival benefit for those ultimately found to have cancer.
KEY POINTS
- Incidentally discovered ovarian cysts are common and most are benign, but a minority can represent ovarian cancer, which is difficult to detect before it has spread and therefore often has a poor prognosis.
- Patients can be reassured if they are postmenopausal and have a simple cyst smaller than 1 cm or if they are premenopausal and have a simple cyst smaller than 5 cm.
- Reassess with yearly ultrasonography in very low-risk situations and with repeat ultrasonography in 6 to 12 weeks if the diagnosis is not clear but is likely benign.
- Refer to a gynecologist in cases of symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing.
- Refer to a gynecologic oncologist for findings worrisome for cancer such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
Physical examination
Vital signs. Fever can indicate an infectious process or torsion of the ovary. A sudden onset of low blood pressure or rapid pulse can indicate a hemorrhagic condition such as ectopic pregnancy or ruptured hemorrhagic cyst.
Bimanual pelvic examination is notoriously inaccurate for detecting and characterizing ovarian cysts. In one prospective study, examiners blinded to the reason for surgery evaluated women under anesthesia. The authors concluded that bimanual examination was of limited value even under the best circumstances.15 Pelvic examination can be even more difficult in patients who are obese, are virginal, have vaginal atrophy, or are in pain.
Useful information that can be obtained through the bimanual examination includes the exact location of pelvic tenderness, the relative firmness of an identified mass, and the existence of nodularity in the posterior cul-de-sac, suggesting advanced ovarian cancer.
Tumor markers
Cancer antigen 125 (CA125) is the most studied and widely used of the ovarian cancer tumor markers. When advanced epithelial ovarian cancer is associated with a markedly elevated level, the value correlates with tumor burden.25
Unfortunately, only about half of early-stage ovarian cancers and 75% to 80% of advanced ovarian cancers express this marker.26 Especially in premenopausal women, there are many pelvic conditions that can falsely elevate CA125. Therefore, its sensitivity and specificity for predicting ovarian cancer are suboptimal. Nevertheless, CA125 is often used to help stratify risk when assessing known ovarian cysts and masses.
The value considered abnormal in postmenopausal women is 35 U/mL or greater, while in premenopausal women the cutoff is less well defined. The lower the cutoff level is set, the more sensitive the test. Recent recommendations advise 50 U/mL or 67 U/mL, rather than the 200 U/mL recommended in the 2002 joint guidelines of the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology.27,28
However, specificity is likely to be lower with these lower cutoff values. Conditions that can elevate CA125 levels include almost anything that irritates the peritoneum, including pregnancy, menstruation, fibroids, endometriosis, infection, and ovarian hyperstimulation, as well as medical conditions such as liver or renal disease, colitis, diverticulitis, congestive heart failure, diabetes, autoimmune diseases, and ascites.
Following serial CA125 levels may be more sensitive than trying to establish a single cutoff value.29 CA125 should not be used as a screening tool in average-risk women.26
OVA1. Several biomarker panels have been developed and evaluated for risk assessment in women with pelvic masses. OVA1, a proprietary panel of tests (Vermillion; Austin, TX) received US Food and Drug Administration approval in 2009. It includes CA125 and four other proteins, from which it calculates a probability score (high or low) using a proprietary formula.
In prospective studies, OVA1 was more sensitive than clinical assessment or CA125 alone.30 The higher sensitivity and negative predictive value were counterbalanced by a lower specificity and positive predictive value.31 Its cost ($650) is not always covered by insurance. OVA1 is not a screening tool.
EVALUATION WITH ULTRASONOGRAPHY
Ultrasonography is the imaging test of choice in assessing adnexal cysts and masses, and therefore it is the best next step after taking a history, performing a physical examination, and obtaining blood work.32 In cases in which an incidental ovarian mass is discovered on computed tomography (CT), further characterization by ultrasonography will likely yield helpful information.
Pelvic ultrasonography can be performed transabdominally or transvaginally. Vaginal ultrasonography gives the clearest images in most patients. Abdominal scanning is indicated for large masses, when vaginal access is difficult (as in virginal patients or those with vaginal atrophy) or when the mass is out of the focal length of the vaginal probe. A full bladder is usually required for the best transabdominal images.
The value of the images obtained depends on the experience of the ultrasonographer and reader and on the equipment. Also, there is currently no widely used standard for reporting the findings33—descriptions are individualized, leading some authors to recommend that the clinician personally review the films to get the most accurate picture.19
Size
Size alone cannot be used to distinguish between benign and malignant lesions. Simple cysts up to 10 cm are most likely benign regardless of menopausal status.2,34 However, in a complex or solid mass, size correlates somewhat with the chance of malignancy, with notable exceptions, such as the famously large sizes of some solid fibromas or mucinous cystadenomas. Also, size may correlate with risk of other complications such as torsion or symptomatic rupture.
Complexity
Simple cysts have clear fluid, thin smooth walls, no loculations or septae, and enhanced through-transmission of echo waves.32,33
Complexity is described in terms of septations, wall thickness, internal echoes, and solid nodules. Increasing complexity does correlate with increased risk of malignancy.
Worrisome findings
The most worrisome findings are:
- Solid areas that are not hyperechoic, especially when there is blood flow to them
- Thick septations, more than 2 or 3 mm wide, especially if there is blood flow within them
- Excrescences on the inner or outer aspect of a cystic area
- Ascites
- Other pelvic or omental masses.
Benign conditions
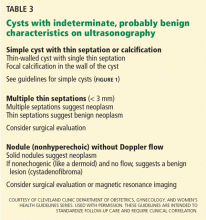
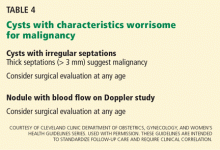
Several benign conditions have characteristic complex findings on ultrasonography (Table 2), whereas other findings can be indeterminate (Table 3) or worrisome for malignancy (Table 4).
Hemorrhagic corpus luteum cysts can be complex with an internal reticular pattern due to organizing clot and fibrin strands. A “ring of fire” vascular pattern is often seen around the cyst bed.
Dermoids (mature cystic teratomas) may have hyperechoic elements with acoustic shadowing and no internal Doppler flow. They can have a complex appearance due to fat, hair, and sebum within the cyst. Dermoid cysts have a pathognomonic appearance on CT with a clear fat-fluid level.
Endometriomas classically have a homogeneous “ground-glass” appearance or low-level echoes, without internal color Doppler flow, wall nodules, or other malignant features.
Fibroids may be pedunculated and may appear to be complex or solid adnexal masses.
Hydrosalpinges may present as tortuous tubular-shaped cystic masses. There may be incomplete septations or indentations seen on opposite sides (the “waist” sign).
Paratubal and paraovarian cysts are usually simple round cysts that can be demonstrated as separate from the ovary. Sometimes these appear complex as well.
Peritoneal inclusion cysts, also known as pseudocysts, are seen in patients with intra-abdominal adhesions. Often multiple septations are seen through clear fluid, with the cyst conforming to the shape of other pelvic structures.
Torsion of the ovary may occur with either benign or malignant masses. Torsion can be diagnosed when venous flow is absent on Doppler. The presence of flow, however, doesn’t rule out torsion, as torsion is often intermittent. The twisted ovary is most often enlarged and can have an edematous appearance. Although typically benign, these should be referred for urgent surgical treatment.
Vascularity
Doppler imaging is being extensively studied. The general principle is that malignant masses will be more vascular, with a high-volume, low-resistance pattern of flow. This can result in a pulsatility index of less than 1 or a resistive index of less than 0.4. In practice, however, there is significant overlap between high and low pulsatility indices and resistive indices in benign and malignant cysts. Low resistance can also be found in endometriomas, corpus luteum cysts, inflammatory masses, and vascular benign neoplasms. A normal (high) resistive index does not rule out malignancy.32,33
One Doppler finding that does seem to correlate with malignancy is the presence of any flow within a solid nodule or wall excrescence.
3D ultrasonography
As the use of 3D ultrasonography increases, studies are yielding different results as to its utility in describing ovarian masses. 3D ultrasonography may be useful in finding centrally located vessels so that Doppler can be applied.32
OTHER IMAGING
Although ultrasonography is the initial imaging study of choice in the evaluation of adnexal masses owing to its high sensitivity, availability, and low cost, studies have shown that up to 20% of adnexal masses can be reported as indeterminate by ultrasonography (Table 1).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is emerging as a very valuable tool when ultrasonography is inconclusive or limited.35 Although MRI is very accurate (Table 1), it is not considered a first-line imaging test because it is more expensive, less available, and more inconvenient for the patient than ultrasonography.
MRI provides additional information on the composition of soft-tissue tumors. Usually, MRI is ordered with contrast, unless there are contraindications to it. The radiologist will evaluate morphologic features, signal intensity, and enhancement of solid areas. Techniques such as dynamic contrast-enhanced MRI (following the distribution of contrast material over time), in- and out-of-phase T1 imaging (looking for fat, such as in dermoids), and the newer diffusion-weighted imaging may further improve characterization.
In one study of MRI as second-line imaging, contrast-enhanced MRI contributed to a greater change in the probability of ovarian cancer than did CT, Doppler ultrasonography, or MRI without contrast.36 This may result in a reduction in unnecessary surgeries and in an increase in proper referrals in cases of suspected malignancy.
Computed tomography
Disadvantages of CT include radiation exposure and poor discrimination of soft tissue. It can, however, differentiate fat or calcifications that may be found in dermoids. While CT is not often used to describe an ovarian lesion, it may be used preoperatively to stage an ovarian cancer or to look for a primary intra-abdominal cancer when an ovarian mass may represent metastasis.32