A 51-year-old woman with dyspnea
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
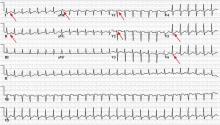
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
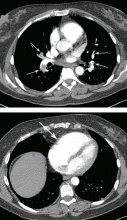
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
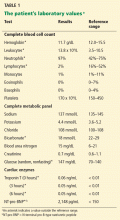
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.