Fecal microbiota transplantation for recurrent C difficile infection: Ready for prime time?
ABSTRACTRecurrent Clostridium difficile infection has been a major challenge for patients and clinicians. Recurrence of infection after treatment with standard antibiotics is becoming more common with the emergence of more-resistant strains of C difficile. Fecal microbiota transplantation is an alternative treatment for recurrent C difficile infection, but it is not yet widely used.
KEY POINTS
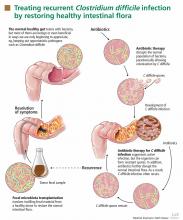
- Fecal microbiota transplantation involves instilling gut microbiota from a healthy donor into the diseased gut of a patient who has recurrent or recalcitrant episodes of diarrhea despite antibiotic treatment for C difficile infection. The instillation can be done via nasogastric tube, endoscope, or enema.
- Donor screening is necessary to prevent transmission of communicable diseases to the recipient.
- Recently published studies indicate that this procedure is effective for treating recurrent C difficile infection. Randomized clinical trials to assess its efficacy and safety are underway.
- The field of microbiota therapy is rapidly progressing. More physicians are learning to embrace the concept of fecal microbiota transplantation, and patients are beginning to overcome the “yuck factor” and accept its benefits.
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
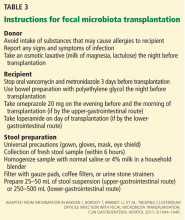
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48