Ascites in a 42-year-old woman
A 42-year-old woman is admitted to the hospital with worsening shortness of breath on exertion, poor exercise tolerance, leg edema, and swelling of the abdomen. Her symptoms have been getting worse over the last 4 months. She reports no history of fever, chills, night sweats, bleeding disorder, joint pain, weight loss, or loss of appetite.
She has type 2 diabetes mellitus and hypothyroidism. She had rheumatoid arthritis but said it was “inactive,” not requiring treatment for the last 18 years. Three months ago, she underwent a total hysterectomy and salpingo-oophorectomy for a complex adnexal mass, biopsy of which revealed a benign mucinous ovarian cyst.
Her current medications include furosemide, levothyroxine, and metformin. She is an ex-smoker with a 7 pack-year history. She drinks a glass of wine on social occasions only. Her family history is unremarkable.
On examination, she is not in distress and she has no fever. She has jugular venous distention of 5 cm, tense ascites, and marked edema of the legs, as well as hyperpigmented patches and erythematous plaques over both shins. Neck palpation reveals no lymphadenopathy or thyromegaly.
Her liver and the tip of the spleen are palpable following paracentesis, once ascitic fluid is removed.
The cardiovascular examination is normal. Chest auscultation reveals decreased breath sounds at the right lung base with bibasilar crackles. No focal neurologic deficit is noted on clinical examination.
Laboratory testing at the time of hospital admission (Table 1) includes a hepatitis panel (negative for exposure to hepatitis A, B, and C) and ascitic fluid studies. Chest radiography shows a right pleural effusion. Echocardiography demonstrates moderate pericardial effusion without tamponade; left and right ventricular function is normal. Cardiac magnetic resonance imaging finds no evidence of pericardial constriction or restrictive cardiomyopathy. Pressures are normal on pulmonary artery catheterization.
FINDING THE CAUSE OF ASCITES
1. What is the most likely cause of ascites in this patient?
- Cirrhosis
- Recent abdominal surgery
- Congestive heart failure
- Abdominal malignancy
- Nephrotic syndrome
The serum-ascites albumin gradient—ie, the serum albumin concentration minus the ascitic fluid albumin concentration—helps determine whether ascites is related to portal hypertension.1 A high gradient (ie, above 1.1 g/dL) is seen in cirrhosis, alcoholic hepatitis, congestive heart failure, vascular occlusion syndromes (eg, Budd-Chiari syndrome), and metastatic liver disease.
From the values in Table 1, our patient’s gradient is 0.8 g/dL, which is considered low. However, we cannot completely rule out cirrhosis as the cause of her ascites because she was taking a diuretic, and diuretics can falsely decrease the gradient. Heart failure is unlikely, based on the results of echocardiography and catheterization. In addition, the 24-hour urinary protein concentration is normal, as is alpha-1 antitrypsin secretion in the stool, ruling out protein-losing nephropathy or enteropathy as the cause of her low albumin and ascites.
A high triglyceride content in her ascitic fluid (> 150 mg/dL) is consistent with chylous ascites, which is seen in patients with previous abdominal surgery or with lymphatic obstruction due to malignancy. A high neutrophil count in the ascitic fluid and a negative culture are also consistent with chylous ascites. However, in this patient, recent surgery as the cause of chylous ascites does not explain the systemic features of hepatosplenomegaly, anemia, thrombocytosis, and low albumin. Moreover, her high C-reactive protein value suggests an ongoing inflammatory process, although her erythrocyte sedimentation rate is not significantly elevated.
Therefore, the most likely cause of ascites in this patient is abdominal malignancy.
WHAT SHOULD BE DONE NEXT?
2. Which of the following studies is reasonable in this patient at this point?
- Serum protein electrophoresis
- Computed tomography (CT) of the chest, abdomen, and pelvis
- Liver biopsy
- Cytologic study of the ascitic fluid
All of these studies would be reasonable and in fact were done in this patient.
Serum protein electrophoresis (Table 2) identified a monoclonal protein band in the immunoglobulin G (IgG) kappa region.
Cytologic study of the ascitic fluid was negative for malignant cells.
Chest CT revealed bilateral pleural effusions, pericardial effusion, and bilateral axillary lymphadenopathy. CT of the abdomen and pelvis was normal, except for ascites, and no pelvic tumor was noted.
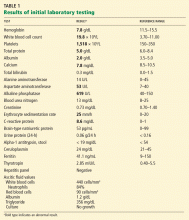
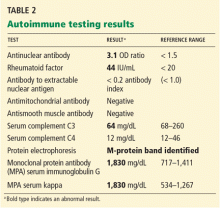
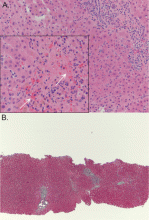
Liver biopsy was done to look for the source of her unexplained ascites with elevated alkaline phosphatase, as all other investigations so far were normal. It revealed mild centrilobular scarring, but the rest of the parenchymal architecture was normal, with no evidence of bridging fibrosis or nodular regenerative hyperplasia (Figure 1).
Transjugular measurement of the hepatic vein pressure revealed a hepatic vein pressure gradient of 9 mm Hg, indicating mild portal hypertension. Venography showed widely patent hepatic and portal veins. Her high inflammatory marker levels could have been caused by smoldering rheumatoid arthritis; however, since the patient has had no joint symptoms for 18 years, this is very unlikely. It is more likely to be caused by a plasma cell disorder, as suggested by a monoclonal protein on electrophoresis.