Locally advanced non–small cell lung cancer: What is the optimal concurrent chemoradiation regimen?
ABSTRACTThe optimal chemoradiation regimen for patients with locally advanced non–small cell lung cancer (NSCLC) has yet to be defined. Disease and patient heterogeneity prevent a “one size fits all” approach to treatment. Concurrent chemoradiation up front is the definitive strategy for patients with unresectable stage III NSCLC; the addition of consolidation chemotherapy following definitive treatment has produced conflicting results with respect to overall survival. Biologic therapies have yet to show value as add-on treatment to chemoradiation.
ATTEMPTS TO IMPROVE RADIOTHERAPY
Methods to improve radiotherapy have centered on evolving radiologic imaging and computer technology, with the objective of enhanced precision of radiation delivery. The routine use of PET in planning radiotherapy allows for dose escalation and control of toxicity.
Radiotherapy dose and outcomes
Three-dimensional (3D) conformal radiation techniques permit the use of higher doses of targeted radiation to spare normal tissue. A meta-analysis of six trials of concurrent chemoradiation therapy concluded that an increased dose of radiation improves both local control and survival.9 A better understanding of normal lung tolerability to radiation therapy is needed to optimize radiation dose.
A clinical trial to test the efficacy of high-dose conformal radiation therapy is in progress. Patients with unresectable stage IIIA or IIIB NSCLC are being randomized to concurrent chemoradiation therapy with carboplatin and weekly paclitaxel with either 74 Gy of radiation in 37 fractions over 7.5 weeks, or 60 Gy of radiation in 30 fractions over 6 weeks. Results will be stratified by radiation therapy technique (3D conformal radiation or intensity-modulated radiation therapy). Following an impressive survival rate (median overall survival: 22.7 months) obtained with the addition of cetuximab to the chemoradiation regimen in the phase 2 Radiation Therapy Oncology Group 0324 trial, an amendment to the design further randomized patients in each radiotherapy group to cetuximab or no cetuximab.10 Those randomized to cetuximab will continue on consolidation therapy with carboplatin, paclitaxel, and cetuximab, while the group randomized to no cetuximab will receive consolidation therapy with carboplatin and paclitaxel only.
Another approach in stage III NSCLC is the use of molecular biomarkers to predict response. Tumor typing for specific molecular sensitivities is generally thought to help predict response to systemic chemotherapy, but within the setting of radiotherapy, patients with a mutation of the epidermal growth factor receptor (EGFR) were found to have more radiosensitive tumors and decreased local recurrence rates than those without the EGFR mutation.11,12 Interactions between systemic therapy and radiation may also prove to be important in response to therapy and prognosis.
ATTEMPTS TO IMPROVE SYSTEMIC THERAPY
A three-arm study compared sequential chemotherapy/radiotherapy, induction chemotherapy followed by concurrent chemoradiation, and concurrent chemoradiation followed by consolidation chemotherapy.14 In the sequential and induction arms, paclitaxel and carboplatin were administered for two cycles prior to radiation therapy; in the consolidation arm, the drugs were given following radiation therapy. The median survival was 16.3 months in the consolidation arm, 12.7 months in the induction arm, and 13.0 months in the sequential arm. The induction and consolidation arms were associated with greater toxicity. The incidences of grade 3/4 esophagitis and pulmonary toxicity were highest in the consolidation arm (28% and 16%, respectively). Although the study was not powered for direct comparison of the three treatment arms, the prolonged median survival for concurrent treatment followed by consolidation chemotherapy adds support to the argument that providing the definitive treatment up front followed by systemically active doses of chemotherapy is the preferred therapeutic approach in stage III NSCLC.
The Southwest Oncology Group (SWOG) study 9504 conducted in patients with stage IIIB NSCLC adds to the evidence of a benefit with consolidation chemotherapy after definitive chemoradiation.15 In this trial, consolidation with docetaxel following concurrent cisplatin-etoposide and radiotherapy extended median overall survival to 26 months.
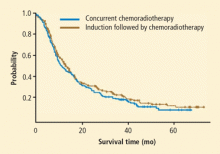
In the Hoosier Oncology Group (HOG) LUN 01-24 study, consolidation with docetaxel after cisplatin-etoposide did not have a survival advantage over cisplatin-etoposide and concurrent radiation alone, but it was associated with increased toxicity in patients with stage III inoperable NSCLC (Figure 2).16
The dose intensity and delivery of consolidation docetaxel were similar in the SWOG 9504 and the HOG LUN 01-24 studies. Although no difference in median survival was observed between the consolidation and observation arms in HOG LUN 01-24, the median survival for the observation arm in this trial was much higher than the 15 months demonstrated with the same concurrent regimen (cisplatin-etoposide and chest radiotherapy) in the SWOG 9019 trial.17 A difference in stage distribution across the two trials might explain the differences in survival in the observation arms.