Stereotactic body radiotherapy for stage I non–small cell lung cancer
ABSTRACTSurgical resection for patients with stage I non–small cell lung cancer (NSCLC) produces high long-term survival rates, but many patients are ineligible for surgery because of medical comorbidity or other factors. Stereotactic body radiotherapy (SBRT) is the standard of care for patients with medically inoperable stage I NSCLC. Studies have reported local control rates with SBRT of about 95% when an adequate radiation dose is used. Lymph node failure averages less than 5%, while distant metastatic recurrence represents the most common site of failure. SBRT is generally safe and well tolerated even by patients with substantial pulmonary comorbidities. On average, lung function tests reveal little or no change from baseline, although individual patients may exhibit changes in pulmonary function after treatment. Most studies report pneumonitis rates of 0% to 5%. Ongoing clinical trials are investigating single-fraction SBRT and evaluating the maximal tolerated dose for centrally located tumors.
SAFETY AND TOLERABILITY
Radiation pneumonitis (an inflammatory complication of radiation frequently characterized by cough, fever, and shortness of breath) is rare—less than 5% in most series. An outlier is a single series that utilized 48 Gy in 4 fractions, a common and well-tolerated dose; the investigators reported a 30% rate of grade 2 through 5 (symptomatic) pneumonitis.18 Pneumonitis was significantly associated with the conformality index, a measure of how tightly the radiation beam is focused on the target tumor, emphasizing the importance of treatment technique on outcomes.
Other notes of caution for patients receiving SBRT include chest wall toxicity and neuropathy. Chest wall toxicity may include a variety of adverse events such as rib fractures, chest wall pain, and skin changes. These events have been described at chest wall radiation doses greater than 30 Gy.19 One study reported brachial plexopathy in 7 of 37 patients who received doses above 100 Gy BED delivered to the brachial plexus.20 Another recent study found that the probability of chest wall toxicity increased as the volume of chest wall receiving a 60 Gy dose increased above 15 to 20 cc.21 Esophagitis and skin reactions are rare except in cases where the patient is being treated for a tumor in extremely close proximity to the esophagus or skin.22
Computed tomography after SBRT often reveals substantial focal fibrosis in the region of high-dose lung radiation.23,24 Despite the often striking radiographic appearance, symptoms are rare and fibrosis may sometimes be mistaken for tumor recurrence. CT images should be read by those experienced in following post-SBRT changes. Findings suspicious for recurrence are typically evaluated by positron emission tomography (PET) followed by biopsy only if PET demonstrates sufficient hypermetabolism.
OPERABLE PATIENTS
Surgical resection is the standard of care for operable patients with lung cancer. Some studies are beginning to examine whether SBRT may also be useful in potentially operable patients. A Japanese study examined outcomes for 87 operable patients who underwent SBRT for stage I NSCLC and who were followed over a 55-month period.25 The local control rate was 92% for T1 tumors, a success rate approaching that of lobectomy. The success rate decreased to 73% for T2 tumors. Five-year overall survival rates were 72% for stage IA and 62% for stage IB, paralleling the surgical experience. Similar early results have been reported from the Netherlands.26 An RTOG study of medically operable patients recently completed enrollment after accruing 33 patients, with final results pending maturation of the data.
A major barrier to the introduction of SBRT to the operable population is the limited nature of the available data; SBRT technology has been implemented only recently and follow-up has been modest, owing to the nature of the medically inoperable population. In addition, it is difficult to determine during the first few months after SBRT which patients will be well controlled. Waiting for response to become apparent is an appropriate strategy for an inoperable patient with no alternatives, but operable patients need a trigger to indicate initiation of salvage therapies.27 In addition, lymph node dissection during surgery often provides information that is essential to tumor staging, and this information might be unavailable for patients treated with SBRT. It is also difficult to weigh the efficacy and tolerability of SBRT against surgical management because the two patient populations are not comparable.
High-risk operable patients
Comparisons of surgery and SBRT for stage I NSCLC are in their infancy and subject to extreme selection bias. Some attempts to create matched populations have demonstrated similar outcomes in matched patients.28,29 Markov modeling suggests improved efficacy for surgery overall, but the model turns in favor of SBRT in patients whose predicted surgical mortality exceeds 4%.30
High-risk operable patients are currently eligible for the American College of Surgeons Oncology group (ACOSOG)/RTOG 0870/Cancer and Leukemia Group B (CALGB) 140503 study; a randomized phase 3 clinical trial that is comparing lobectomy versus sublobar resection for small (< 2 cm) peripheral NSCLC. This study should help to clarify how this higher-risk patient group should be managed.
CLEVELAND CLINIC EXPERIENCE
At Cleveland Clinic, more than 700 patients with stage I NSCLC have been treated with SBRT since 2003. Peripheral tumors are typically treated with a radiation dose of 60 Gy in 3 fractions spaced over 8 to 14 days, or alternatively 30 Gy to 34 Gy in a single fraction. Occasional large tumors near the chest wall or spinal cord are treated with doses up to 50 Gy in 5 fractions over 5 consecutive days. For central tumors, radiation dose regimens include 50 Gy (5 fractions over 5 consecutive days) or 60 Gy (8 fractions over 10 days), depending upon tumor size and proximity to critical structures.
SUMMARY AND CONCLUSIONS
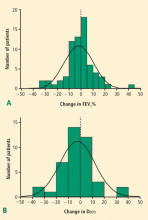
Many patients with NSCLC are ineligible for surgery because of COPD, cardiovascular disease, or other conditions associated with unacceptably high perioperative risk. SBRT is the standard of care for patients with medically inoperable stage I NSCLC. Modern standard radiation doses are typically between 50 to 60 Gy in 3 to 5 fractions. Local control rates in excess of 90% to 95% have been reported with these doses. SBRT is generally well tolerated by patients with both peripheral and centrally located tumors. On average, lung function is not substantially altered by SBRT, although individual patients may exhibit increased or decreased FEV1 and Dlco values after treatment. Pneumonitis has been relatively rare in most studies, with typical rates of 0% to 5%. SBRT has been shown to produce reasonable rates of local control in potentially operable patients, although data are extremely limited in this population and there are important questions about salvage therapy and postprocedural evaluation in these patients. Several ongoing clinical trials are continuing to define the efficacy and safety of different radiation dosing procedures for patients with inoperable NSCLC.