Biologic agents in the treatment of granulomatosis with polyangiitis
ABSTRACTGranulomatosis with polyangiitis (GPA) is a type of vasculitis that affects the respiratory tract and kidneys. Without treatment, half of patients die within 6 months. Standard therapy (a daily combination of cyclophosphamide and glucocorticoids) can induce remission, but the duration is short and treatment is plagued by serious morbidity. Advances in understanding the potential target of cyclophosphamide—B cells, that indirectly give rise to antineutrophil cytoplasmic antibodies (ANCA)—led to a new B-cell–targeted strategy. We administered rituximab, an anti–B-cell agent, to patients with severe GPA and microscopic polyangiitis. Overall, rituximab matched the efficacy of cyclophosphamide in inducing remission and was superior in patients with relapsing disease. The timing of re-treatment can be individualized based on patients’ B-cell counts and ANCA levels in patients with chronically relapsing GPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
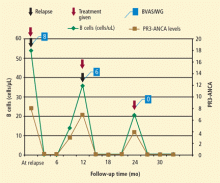
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.