Monitoring patients with vasculitis
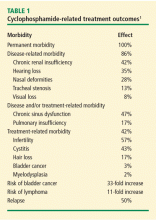
ABSTRACTGranulomatosis with polyangiitis is a common form of small-vessel vasculitis, remarkable for its tendency toward multisystem manifestations. Standard induction treatment calls for the use of low-dose daily cyclophosphamide (CYC) and glucocorticoids. Treatment goals for newly diagnosed patients include increased survival, induction of remission, reduction of relapse frequency, and minimization of treatment toxicity. Induction and maintenance treatments with CYC, glucocorticoids, and other immunosuppressive therapies improve the disease course, but relapse- and treatment-related toxicity and infections demand consistent, patient-specific monitoring.
Granulomatosis with polyangiitis (GPA), is one of the most common types of small-vessel vasculitis, with an estimated prevalence in the United States of 3 per 100,000 people. It is distinguished from other necrotizing vasculitides by its tendency to affect the upper and lower respiratory system and the kidneys. Despite the success of induction and maintenance treatments with cyclophosphamide (CYC), glucocorticoids, and less toxic immunosuppressive alternative therapies in improving the disease course, significant treatment-related toxicities and frequent disease relapses demand stringent patient-specific monitoring in order to provide early treatment of relapses and prevent or decrease morbidity.
SMALL-VESSEL VASCULITIS MANAGEMENT OVERVIEW
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis, or WG) is an antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis that often affects the respiratory system and kidneys across a broad spectrum of clinical presentations, from mild through life-threatening disease. Patients with severe disease present with significant multisystem manifestations, which, in addition to the respiratory system and kidneys, may involve the joints, eyes, and other organs.
,Managing patients diagnosed with systemic small-vessel vasculitides such as GPA and microscopic polyangiitis (MPA) is an inexact science. The goals of treatment are to increase survival, induce and maintain remission, reduce relapses, and minimize treatment-related toxicity. Inducing and maintaining remission have become realistic goals because of the availability of medications that prolong life. On the other hand, extended periods of treatment associated with prolonged life increase the risk of treatment-related toxicity in patients who are inadequately monitored.
MONITORING CONSIDERATIONS
Achieving treatment goals requires long-term monitoring of both disease activity and treatment-related toxicities, with constant adjustments to meet the needs of the individual patient and address the often rapidly changing disease and treatment course. The monitoring protocol consists of regularly scheduled follow-up office visits, urine sediment analyses at every office visit whether or not the patient has relapse symptoms, laboratory tests at regular intervals as indicated by the patient’s medication plan and disease presentation, additional tests such as lung computed tomography (CT), and patient education regarding new symptoms and the frequency of office visits. A consistent monitoring strategy will help detect a relapse before it can produce more severe morbidity, identify treatment-related complications, and—equally important—identify the achievement of remission. An example of the consequences of inconsistent monitoring is presented in “Relapse in a nonadherent patient.”
Because there is no definitive cure for small-vessel vasculitis, relapse is always a possibility. The early diagnosis and treatment of relapse may prevent or decrease morbidity from disease, but strict monitoring is needed to identify relapse and initiate treatment before morbidity occurs (see “Relapse in a patient with new symptoms”). Repeat induction therapy following a relapse introduces risk of drug toxicity and requires careful monitoring, as does long-term maintenance therapy.
In addition to induction and maintenance therapy, several other situations, including prior therapeutic complications, serum creatinine levels, and risk of cardiovascular disease, require special monitoring attention.

Induction therapy: monitor response

Response to treatment during induction must be monitored to identify whether remission is achieved. Induction monitoring requires complete assessment of organ-system involvement at every visit with tools such as the Birmingham Vasculitis Activity Score (BVAS) and, when appropriate, the BVAS/WG. If new or worsening symptoms develop during induction therapy, then the patient needs assessment for continued disease activity as well as treatment complications such as infections related to immunosuppressive therapy.
During induction therapy with daily oral CYC, monitoring should include weekly complete blood cell counts to ensure early identification of leukopenia and other cytopenias. The risk of morbidities increases with the cumulative dose, so a stable blood count for 2 months does not obviate the risk of leukopenia. If persistent hematuria is present without cellular casts, cystoscopy is indicated to look for signs of hemorrhagic cystitis. Prophylaxis against Pneumocystis jirovecii is recommended in all patients who receive immunosuppressive therapy. Finally, bone density measurements should be done at baseline.