Pulmonary disease in small-vessel vasculitis
ABSTRACTDiagnosis of the pulmonary manifestations of small-vessel vasculitis requires attention to detail, judicious use of imaging technology, and awareness of disorders that can mimic or masquerade as pulmonary vasculitis. Treatment should begin with pharmacologic intervention to manage the underlying inflammatory disorder. Dilation procedures and, in rare cases, surgery may be needed to resolve airway stenosis.
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
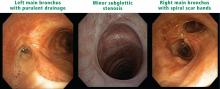
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.