Use of chemodenervation in dystonic conditions
ABSTRACTDystonia, an uncommon movement disorder that causes sustained muscle contractions and painful body positions, is a difficult diagnostic challenge; misdiagnosis is common. Classification may include etiology, area of physical involvement, or age of onset. Bodily distribution is varied, and dystonias can present as primary (genetic) or secondary (caused by other disease processes or use of neuroleptic drugs). Although there is no cure, the use of botulinum toxins for chemodenervation provides symptomatic relief and is considered the treatment of choice in focal dystonia. The dose of botulinum toxin may be titrated to provide significant relief for 12 weeks or more.
SYMPTOMATIC TREATMENT WITH CHEMODENERVATION
In the absence of a cure, treatment options for dystonia are necessarily symptomatic and supportive. Titratable chemo denervation agents are injected directly into the muscle or motor nerve, temporarily weakening the local muscle and easing dystonia symptoms. Chemo denervation agents include phenol, ethyl alcohol, and botulinum toxin types A (BTX-A; onabotulinumtoxinA, abo botulinumtoxinA, and incobotulinumtoxinA) and B (BTX-B; rimabotulinum toxinB).
Phenol and ethyl alcohol injections targeted perineurally or as a motor point block have been employed for dystonia and cause nonselective tissue destruction, muscle necrosis, and highly variable durations of response. Perineural microcirculation may be damaged, possibly leading to long-term defects.
Clostridium botulinum bacteria produce seven serologically distinct neuroparalytic toxins. They are the most powerful such toxins currently known and temporarily prevent acetylcholine vesicles from docking into the presynaptic neuromuscular junction. Use of BTX-A for treatment of dystonia was recommended in a National Institutes of Health consensus statement in 1990.9 It has been studied for a variety of dystonias, including blepharospasm, hemifacial spasm, laryngeal dystonia, oromandibular dystonia, and cervical dystonia, among other focal dystonias. Lew et al reported in 1997 on the successful use of BTX-B for cervical dystonia in a double-blind, single-treatment study,10 and confirmatory studies followed.11,12
Varying indications for botulinum toxin
US Food and Drug Administration–approved indications for the toxins vary. The three BTX-A products and the single BTX-B product are approved for the treatment of cervical dystonia in adults to reduce the severity of abnormal head position and neck pain. OnabotulinumtoxinA is approved for treatment of blepharospasm and strabismus associated with dystonia; and incobotulinumtoxinA is approved for blepharospasm in patients who have previously been treated with onabotulinumtoxinA. BTX-A has also been found to be safe and effective for the management of focal dystonias. These botulinum toxin agents are not equivalent in dosing units, so caution must be observed when switching brands.
Patients selected to receive BTX for dystonia should meet three criteria:
- The dystonia should interfere with their functioning, comfort, or care to the degree that causes impairment and affects activities of daily living;
- Focal weakening following administration of the drug should not decrease their level of function; and
- The patient should understand that use of BTX may not completely address positioning, posturing, or secondary deformities.
Contraindications include pregnancy, lactation, comorbid neuromuscular disease (eg, amyotrophic lateral sclerosis or myasthenia gravis), and use of an aminoglycoside.
The need for BTX therapy should be reevaluated prior to each treatment; clinical benefit lasts 3 months or more. Electromyography may facilitate the location of target muscles, particularly since involved musculature may not be palpable and is often not superficial.13 In-office tools that help document baseline and posttreatment results, including videotaping dystonic limb movements and the use of rating scales, can be important for evaluating the patient’s progress.14
Relief for cervical dystonia
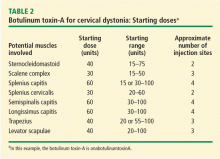
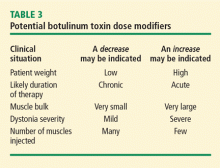
The treatment of choice for focal dystonias and focal aspects of generalized dystonia is BTX. Both BTX-A and BTX-B offer effective palliative treatments for cervical dystonia by improving neck position, reducing pain, and decreasing disability in sufferers.11,15–18 The BTX solution is injected directly into the dystonic muscle at several locations, temporarily weakening the overactive muscle. The BTX dose is approximately proportional to the size of the muscle, although smaller muscles typically responsible for precision movement may require a relatively larger dose (Table 2). Doses may be modified according to clinical factors such as muscle bulk and severity of dystonia (Table 3).
Relief following BTX injection for cervical dystonia occurs about 1 week later, with the greatest effect seen at about 2 to 6 weeks following injection; relief may last 12 to 16 weeks. Reinjections are not normally administered prior to 12 weeks’ duration in order to reduce the possibility of antibody formation. Concomitant interventions addressing depression and anxiety may have a significant effect on overall quality of life.19 Patients may also try several sensory tricks, called gestes antagoniste, which may temporarily reduce or alleviate the dystonia. However, these tactile procedures—such as placing a hand on top of the head—lose their effectiveness over time.
Treatment of blepharospasm, focal limb dystonia
The use of BTX-A for blepharospasm is a significant improvement over the former clinical reliance on various oral medications, which, with the exception of baclofen, proved largely ineffective.20 Surgical treatments result in damage to muscular and nervous tissues, and so are reserved only for nonresponders to BTX-A therapy.21
BTX-A can provide effective relief and is the treatment of choice for focal limb dystonias.22 Goals of treatment include functional improvement, correction of abnormal posture, and relief from discomfort. Although a variety of oral medications may also be prescribed, drug toxicity and adverse effects can outweigh the benefit and are usually only used in cases of severe dystonia. Oral medications used for limb dystonia include anticholinergics, dopamine agonists and antagonists, baclofen, clonazepam or other benzodiazepines, and muscle relaxants.
Antibodies may bind to the drug in a small percentage of patients who regularly receive injections of BTX, rendering additional injections of that specific serotype of BTX ineffective. This immunoresistance can be avoided if clinicians inject only the smallest quantity of BTX that achieves clinical efficacy, avoid administering booster injections before the end of the minimum 12-week lockout period, and extend the period between treatments as long as possible. If immunoresistance does occur, the BTX should be exchanged for a different serotype.
Testing for nonresponse
Patients are said to be nonresponders to BTX therapy if at 4 to 6 weeks following injection they show no reduction in muscle tone. A functional test for nonresponse is to inject a small amount of BTX into either the frontalis or sternocleidomastoid muscle prior to starting treatment; asymmetric weakness demonstrates a response, indicating that either injection technique or muscle selection is the problem. In addition to the development of neutralizing antibodies, other possible reasons for nonresponse include a dose that is too low or an alteration in the pattern of muscles involved in the dystonic movement.