2012–2013 Influenza update: Hitting a rapidly moving target
ABSTRACTFrom the deadly 2009 influenza A H1N1 pandemic to the looming threat of bird flu H5N1, the recent outbreak of swine flu H3N2v at agriculture fairs, and the emergence of drug-resistant H1N1, we are constantly challenged by influenza viruses. Vaccination remains the main strategy for prevention. With the knowledge gained from past pandemics, an adequate vaccine supply, and an updated preventive strategy, we are in a better position to face the challenge.
KEY POINTS
- A recent outbreak of swine flu in children exposed to pigs at agricultural fairs is unprecedented. Seasonal influenza vaccine does not protect against this strain, designated H3N2v. The neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza) are the drugs of choice for treatment.
- A highly lethal bird flu, designated H5N1, is still a pandemic threat. In the event of an outbreak, an inactivated whole-virus vaccine is available.
- A community outbreak of oseltamivir-resistant H1N1 in Australia sounded an alarm for a potential drug-resistant flu epidemic. Inhaled zanamivir would be the only effective therapy available in the event of such an epidemic.
- An emerging new antiviral drug is effective against oseltamivir-resistant influenza.
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
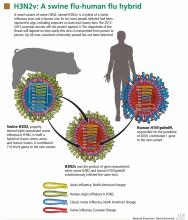
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21