Imaging for autonomic dysfunction
ABSTRACT
Direct visualization of heart-brain interactions is the goal when assessing autonomic nervous system function. Cortical topology relevant to neuroimaging consists of the cingulate, insula, and amygdala, all of which share proximity to the basal ganglia. Significant cardiac effects stemming from brain injury are well known, including alteration of cardiac rhythms, cardiac variability, and blood pressure regulation; in some instances, these effects may correlate with neuroimaging, depending on the region of the brain involved. It is difficult to achieve visualization of areas within the brainstem that govern autonomic responses, although investigators have identified brain correlates of autonomic function with the use of functional magnetic resonance imaging and electrocardiographic data obtained simultaneously. The potential utility of brain imaging in sick patients may be limited because of challenges such as the magnetic resonance imaging environment and blunted autonomic responses, but continued investigation is warranted.
The autonomic nervous system (ANS), composed of the sympathetic and parasympathetic nervous systems, governs our adaptation to changing environments such as physical threats or changes in temperature. It has been difficult to elucidate this process in humans, however, because of limitations in neuroimaging caused by artifacts from cardiorespiratory sources. This article reviews structural and functional imaging that can provide insights into the ANS.
STRUCTURAL IMAGING
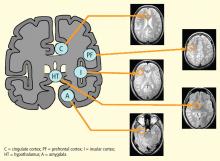
The two main subcortical areas of interest for imaging are the lateral hypothalamic area and the paraventricular nucleus, but visualization is difficult. The hypothalamus occupies a volumetric area of the brain no larger than 20 voxels; individual substructures of the hypothalamus therefore cannot easily be viewed by conventional imaging. The larger voxel size of functional MRI (fMRI) mean that fMRI of the hypothalamus can display 1 voxel at most.
Most brainstem nuclei are motor nuclei that affect autonomic responses, either sympathetic or parasympathetic. These nuclei are difficult to visualize on conventional MRI for two reasons: the nuclei are small, and may be the size of only 1 to 2 voxels. More important, MRI contrast between these nuclei and surrounding parenchyma is minimal because these structures “blend in” with the surrounding brain and are difficult to visualize singly. Examples of these major brainstem sympathetic nuclei are the periaqueductal gray substance, parabrachial nuclei, solitary nucleus, and the hypothalamospinal tract; examples of the major brainstem parasympathetic nuclei are the dorsal nucleus of the vagus nerve and the nucleus ambiguus.
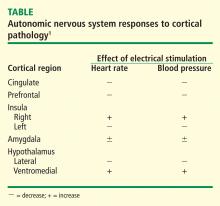
The areas of the ANS under cortical control are more integrative, with influence from higher cognitive function—for example, the panic or fear associated with public speaking. Regions of subcortical control involve the basal ganglia and hypothalamus, which regulate primitive, subconscious activity, such as “fight or flight” response, pain reaction, and fear of snakes, all of which affect multiple motor nuclei. Several specific sympathetic and parasympathetic motor nuclei directly affect heart rate and blood pressure and act as relay stations for sensory impulses that reach the cerebral cortex.
NEUROLOGIC PROCESSES AND CARDIAC EFFECTS
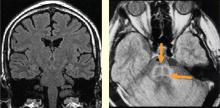
MS is classically a disease of white matter, although it can also affect gray matter. Autonomic dysfunction is common, affecting as many as 50% of MS patients with symptoms that include orthostatic dizziness, bladder disturbances, temperature instability, gastrointestinal disturbances, and sweating.1–4 The effect of autonomic dysfunction on disease activity is unclear. Multiple brainstem lesions are evident on MRI, and may be linked to cardiac autonomic dysfunction. The variability of MS contributes to the difficulty of using imaging to identify culprit lesions.
Stroke causes autonomic dysfunction, with the specific manifestations dependent on the region of the brain involved. In cases of right middle cerebral artery infarct affecting the right insula, an increased incidence of cardiac arrhythmias, cardiac death, and catecholamine production ensues.5–7 Medullary infarcts have been shown to produce significant autonomic dysfunction.8,9
Ictal and interictal cardiac manifestations in epilepsy often precede seizure onset.1 Common cardiac changes are ictal tachycardia or ictal bradycardia, or both, with no clear relationship to the location or type of seizure. Evidence suggests that heart rate variability changes in epilepsy result from interictal autonomic alterations, including sympathetic or parasympathetic dominance. Investigation of baroreflex responses with temporal lobe epilepsy has uncovered decreased baroreflex sensitivity. There is no reliable correlation between sympathetic or parasympathetic upregulation or downregulation and brain MRI findings, however.
Autonomic dysfunction in the form of orthostatic hypotension has been documented in patients with mass effect from tumors, for example posterior fossa epidermoid tumors, wherein tumor resection results in improved autonomic function.10