Bronchial thermoplasty: A new treatment for severe refractory asthma
ABSTRACTBronchial thermoplasty was recently approved for treating severe refractory asthma that is not well controlled by high-dose inhaled corticosteroids and long-acting bronchodilator therapy. This article reviews its indications, evidence of efficacy, and protocols.
KEY POINTS
- Bronchial thermoplasty involves the application of radiofrequency energy to the airways distal to the mainstem bronchi down to airways as small as 3 mm in diameter.
- Treatments are done in three separate sessions, with careful monitoring before and after for respiratory complications that can occur in severe asthma. Airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure, thus requiring close patient follow-up.
- In clinical trials, including a randomized trial in which the control group underwent sham thermoplasty, bronchial thermoplasty had an acceptable safety profile while improving asthma quality-of-life scores, symptoms, and health care utilization.
AIR2: A randomized, double-blind trial
The latest trial of this new therapy in severe asthma was the AIR2 trial.6 A major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty, allowing the trial to be truly double-blinded. (The bronchoscopy team knew which patients got which treatment, but the patients and the study physicians following them did not).
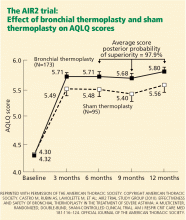
The primary outcome was the change in AQLQ score from baseline at 6, 9, and 12 months. Secondary outcomes included absolute changes in the asthma control scores, symptom scores, peak flows, rescue medication use, and FEV1.
The randomized groups (196 patients in the thermoplasty group and 101 in the sham treatment group) were well matched, and more than 80% in each group met the American Thoracic Society criteria for severe refractory asthma.
On the AQLQ, a change of more than 0.5 is considered clinically meaningful. Interestingly, there was a significant and clinically meaningful improvement in AQLQ in 64% of the sham treatment group, highlighting the placebo effect in asthma treatment.19 However, a larger proportion (79%) of the treated group had a clinically meaningful improvement on the AQLQ than in the sham treatment group.
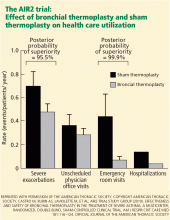
Adverse events occurred in both groups; however, during the treatment phase, 16 patients in the bronchial thermoplasty group needed to be hospitalized for respiratory symptoms including worsening asthma, atelectasis, lower respiratory tract infections, decreased FEV1, and an aspirated tooth. One episode of hemoptysis required bronchial artery embolization. In contrast, only two patients in the sham treatment group needed hospitalization.
Therefore, this trial showed that patients with severe asthma treated with bronchial thermoplasty had a long-term improvement in quality of life and needed less health care.6
Translating these trials into practice
To summarize, these clinical trials showed that bronchial thermoplasty was feasible, was relatively safe, and produced better clinical outcomes in patients with severe asthma when medical therapies did not control their symptoms.
In practice, patient selection is likely to be important. A key question will be, Does the patient truly have severe refractory asthma, or is the patient not taking his or her medication? Adherence to therapy should be evaluated.
In addition, patients need to be observed and monitored closely during and after the treatment period, as airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure. About 80% of all study patients had multiple symptoms of asthma and other symptoms in the treatment period. Rarely did these symptoms result in hospitalization, but they were more common in the treatment group in the AIR2 trial.
Long-term studies have evaluated the duration of effect and the safety of bronchial thermoplasty, and outcomes appear favorable.20,21
WHY DOES IT WORK?
The role of airway smooth muscle in asthma is yet to be fully elucidated. The trials outlined here showed that although asthma is a disease of the airways, including the small airways, treatment of airways 3 mm or larger improves asthma symptoms, quality of life, and health care utilization.6 Thus, the role of airway smooth muscle in asthma and as a target of therapy has not previously been fully realized.21
Early investigations into the mechanisms of airflow obstruction and airway resistance found that 75% of postnasal resistance occurs in the first six to eight generations (ie, branchings) of the airways, indicating that larger airways are involved.22 (The number of generations varies depending on the size of the person but it typically is 10 to 12.) Findings from the study in dogs introduced the idea that smooth muscle alterations contributed to the changes in airway resistance, and that subtle changes in airway smooth muscle could clinically benefit asthma patients.1
The speculated purpose of the airway smooth muscle layer is to support the airway, allow gas exchange, propel mucus for clearance, defend the airway, enhance cough, and promote lymphatic flow. However, the airway smooth muscle layer may also be vestigial. In asthma, airway smooth muscle adds to bronchoconstriction and hyperresponsiveness, and has a role in mediating inflammation and airway remodeling.21 No definitive studies have shown that eliminating airway smooth muscle greatly inhibits normal airway function.18
What exactly does thermoplasty do to the smooth muscle? Studies in smooth muscle from cows showed that high temperatures directly disrupt the actin-myosin interaction, likely through denaturation of motor proteins.23 This immediate loss of muscle cell function is not likely to be the result of apoptosis, autophagy, or necrosis, or mediated by heat-shock proteins, in view of the relatively quick muscle response and lack of progressive changes. Tissue responsiveness is substantially reduced a few seconds after application of 60°C of heat and is subsequently abolished within 5 minutes after treatment.23
The intervention appears to be dose-dependent. Responsiveness to cholinergic stimulation is lessened by treatment, and the desired effect is seen within seconds and does not progress.
Therefore, we can surmise that disruption of myosin function is likely the mechanism of the therapeutic effect, breaking the cascade of airway smooth muscle spasm. Now that we know about the airway smooth muscle as a possible target of therapy, and that it may play only a vestigial role, we can think about other therapies that focus on it.18,23