Malignant bowel obstruction: Individualized treatment near the end of life
ABSTRACTMalignant bowel obstruction requires a highly individualized approach, tailored to the patient’s medical condition, prognosis, and goals of care. Surgery should not be routinely done. Less-invasive approaches such as gastric and colonic stenting are useful.
KEY POINTS
- Combinations of analgesics, antisecretory drugs, and antiemetics can provide acceptable symptom relief.
- A venting gastrostomy should be considered if drug therapy fails to reduce nausea and vomiting to an acceptable level.
- A nasogastric tube should be used only as a temporizing measure, until symptoms are controlled medically or a venting gastrostomy is placed.
- Total parenteral nutrition is beneficial only in patients with intermediate life expectancy who may otherwise die of starvation rather than the cancer itself.
Malignant bowel obstruction occurs in 5% to 51% of women with ovarian cancer and in 10% to 28% of patients with gastrointestinal cancer, predominantly in the advanced stages.1 Median survival after its onset ranges from 30 to 90 days.2–5
Its symptoms are challenging to manage, since nausea, vomiting, colic, and abdominal pain, which are common, cause significant physical distress and demoralization. The decision whether to correct it with surgery requires an individualized approach and a clear understanding of the goals of care and expected survival in the individual patient.
This review focuses on the management of inoperable malignant bowel obstruction and includes discussion of hydration, nutrition, and endoscopic palliative options.
WHAT ARE THE DIFFERENT TYPES OF OBSTRUCTION?
Bowel obstruction may be mechanical or functional, partial or complete, and may occur at one or at many sites. Tumors can impair bowel function in several ways6–8:
- Intraluminal tumors can occlude the lumen or act as a point of intussusception.
- Intramural tumors can extend to the mucosa and obstruct the lumen or impair peristalsis.
- Mesenteric and omental masses or malignant adhesions can kink or angulate the bowel, creating an extramural obstruction.
- Tumors that infiltrate into the mesentery, bowel muscle, or the enteric or celiac plexus can cause dysmotility.
Cholangiocarcinoma, pancreatic carcinoma, and gallbladder carcinoma are the most common tumors causing duodenal obstruction.9 Distal obstruction is caused mainly by colon and ovarian cancer.
Obstruction can be due to treatment
In a minority of patients, obstruction is unrelated to the cancer and is instead due to adhesions arising from surgery, radiation therapy (causing enteritis and strictures), desmoplastic reactions to intraperitoneal chemotherapy, torsion, or internal hernias.10–12
In rare cases, a patient has intestinal pseudo-obstruction from paraneoplastic destruction of enteric neurons, or severe ileus from anticholinergic or sympathomimetic drugs, as seen with acute colonic pseudo-obstruction (Ogilvie syndrome).13
Physiologic reactions to obstruction
Malignant bowel obstruction stimulates gastric, biliary, pancreatic, and intestinal secretions, decreases intraluminal sodium and water reabsorption, and increases mucosal sodium and water secretion.6,14 In response to the obstruction, peristalsis increases, and prostaglandin, vasoactive intestinal peptide, and nociceptive mediators are released. Vasoactive intestinal polypeptide perpetuates a cycle of secretion, distention, and contraction that leads to intestinal hyperemia, bowel edema, and accumulation of fluid in the lumen.8,10,11,15
Signs and symptoms depend on the site
The site of obstruction determines the signs and symptoms patients experience.7,14 Obstructions high in the gastrointestinal tract are associated with greater symptoms but fewer signs than colonic obstructions.1 Patients with proximal small-bowel obstruction have more severe nausea and a greater number of episodes of emesis, but they have relatively normal plain radiographs of the abdomen, which do not have the characteristic air-fluid levels commonly seen with distal small-bowel obstruction.
Most malignant obstructions remain partial, but increasing abdominal distention, worsening nausea, vomiting, abdominal pain, and obstipation over 1 to 2 weeks1 suggest progression to complete obstruction.
IMAGING TESTS FOR MALIGNANT BOWEL OBSTRUCTION
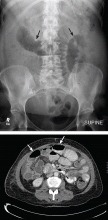
What is the value of plain radiography?
Despite these limitations, plain radiography is useful in assessing constipation and its severity as a potential cause of symptoms, and thus it remains an important initial imaging study in almost all patients with suspected malignant bowel obstruction.17,18 It is also used to assess response to treatment.
When do you need contrast radiographs?
Contrast radiography (barium swallow or barium or Gastrograffin enema) is helpful in patients with symptoms of dysmotility from suspected bowel obstruction. It defines the site or sites of obstruction and the extent of the obstruction with a fair degree of accuracy.7,19 Single-contrast studies, if positive, exclude opioid-induced bowel dysfunction or pseudo-obstruction in 83% of patients, with a sensitivity and specificity of 96% and 98%, respectively.8,20 Small-bowel follow-through with barium is more appropriate for low-grade obstructions or for symptomatic patients with a normal kidney, ureter, bladder radiograph.19
However, contrast radiography is limited by the patient’s ability to swallow barium or water-soluble contrast agents, and it can worsen nausea or vomiting.17,18 Also, barium is not absorbed systemically and may interfere with subsequent radiologic studies. Large volumes of contrast agents increase the risk of aspiration pneumonia in patients with poorly controlled nausea and can lead to severe impaction proximal to the obstructed site.8