Interpreting the estimated glomerular filtration rate in primary care: Benefits and pitfalls
ABSTRACTAs several equations have been developed for estimating the glomerular filtration rate (GFR), many laboratories are now reporting the GFR automatically, and primary care providers are left trying to interpret the results and put them into the context of patient care. Therefore, it is important that health care professionals understand how to interpret the estimated GFR value and how to recognize when the estimate may not be accurate.
KEY POINTS
- Chronic kidney disease must be detected in its early stages so that measures can be taken to detect its complications and to delay its progression to kidney failure.
- The creatinine concentration is an imperfect marker of renal function and should not be used by itself in assessing renal function.
- Formulas for estimating the GFR from the serum creatinine level along with other easily obtained variables continue to be refined.
- Primary care physicians and nephrologists need to collaborate to provide the optimal care for patients with chronic kidney disease.
ESTIMATING THE GFR
Measuring 24-hour creatinine clearance
Measuring 24-hour creatinine clearance involves measuring the concentrations of creatinine in the serum and the urine and the volume of urine excreted in 24 hours.
The 24-hour creatinine clearance was long considered the best alternative to the serum creatinine concentration for assessing kidney function, as it adjusts for changes in the creatinine concentration by taking into account creatinine’s excretion in the urine. However, 24-hour urine collection is burdensome for the patient, and the results are not always reliable because of variations in collection technique. Also, using the creatinine clearance does not resolve problems with using the serum creatinine concentration, such as tubular secretion and overestimation of GFR.
In an effort to more easily estimate GFR from blood tests alone, efforts to develop mathematical equations that more closely estimate GFR began over 40 years ago. These equations take into account factors such as age, sex, and ethnicity. The best known of these are the Cockcroft and Gault10 and the MDRD equations.5
The Cockcroft-Gault equation
The Cockcroft-Gault equation is fairly simple, using serum creatinine, ideal body weight, and an adjustment factor for sex. Its main drawbacks are that it was developed to model creatinine clearance, itself an imperfect estimation of GFR, and it depends heavily on the accuracy of the value for “lean” body weight used in the equation.
The MDRD equation
The MDRD equation has now largely replaced the Cockcroft-Gault equation. Developed using iothalamate GFR measurements, it therefore estimates GFR rather than the less-accurate creatinine clearance. Also, it is normalized to a standard body surface area (1.73 m2), obviating the need to determine ideal body weight.
Since the estimated GFR can often be calculated using data available in most electronic medical record systems, it can be reported directly with any laboratory report that includes a serum creatinine value.
The main drawback of the MDRD equation is that it tends to underestimate GFR at higher ranges of kidney function, ie, higher than 60 mL/min/1.73 m2).3,11
The CKD-EPI equation
The Chronic Kidney Disease Epidemiology Collaboration study (CKD-EPI) equation,12 published in 2009, is expected to eventually replace the currently used MDRD equation, as it performs better at higher ranges of GFR.
Although the CKD-EPI equation still lacks precision and accuracy, it underestimates GFR to a lesser degree than the MDRD equation in patients with preserved renal function. Also, it was developed with the objective of reporting a specific value even when the estimated GFR is greater than 60 mL/min/1.73 m2. (In contrast, when laboratories use the MDRD equation, the recommendation is to report any value above this level as “greater than 60 mL/min/1.73 m2”).
A limitation of all equations that use the serum creatinine concentration to assess kidney function is the assumption that creatinine production is both stable over time and similar among patients. As a result, these equations should not be used in situations in which renal function is changing rapidly, such as in acute kidney injury. Also, they should be used with caution in patients at the extremes of body mass, since they underestimate GFR in very muscular patients (eg, as in case 2) and overestimate GFR in very small patients (eg, as in case 1).
Calculators for estimating the GFR using these equations are available on many Web sites (see www.kidney.org/professionals/kdoqi/gfr_calculator.cfm).
SCREEN EVERYONE AT RISK
In general, anyone at higher risk of chronic kidney disease should be screened for it. This group includes US minorities and patients with hypertension, cardiovascular disease, and diabetes mellitus, among others.13 Screening includes an assessment of estimated GFR and urinalysis for proteinuria or hematuria.
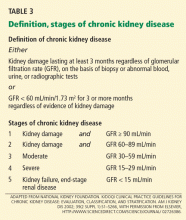
CHRONIC KIDNEY DISEASE DEFINED: DAMAGE AND DURATION
The kidney damage can be either parenchymal renal damage independent of GFR (for example, cystic disease, glomerular hematuria, or proteinuria) or depressed GFR independent of evidence of parenchymal renal disease (an estimated GFR of less than 60 mL/min/1.73 m2).
The duration component requires that the abnormality be present for at least 3 months (ie, chronic).