Dronedarone for atrial fibrillation: How does it compare with amiodarone?
ABSTRACTDronedarone (Multaq), an analogue of amiodarone (Cordarone), was designed to cause fewer adverse effects than the parent compound. Studies have indeed shown dronedarone to be safer than amiodarone, but less effective. Its official indication is to reduce the risk of hospitalization in patients with paroxysmal or persistent atrial fibrillation or atrial flutter and other cardiovascular risk factors, reflecting the parameters of its effectiveness in clinical trials.
KEY POINTS
- Patients with persistent or paroxysmal atrial fibrillation are candidates for dronedarone therapy if they are in sinus rhythm or will be cardioverted soon after starting. This drug is not indicated for the acute management of atrial fibrillation, for example, in the emergency department.
- Dronedarone is an option if a patient cannot tolerate amiodarone or has an underlying condition such as pulmonary or thyroid disease that is a contraindication to amiodarone.
- Dronedarone is contraindicated in patients with significant left ventricular dysfunction or heart failure with recent decompensation.
- The ultimate role for dronedarone is yet to be defined. Little evidence exists as to whether it will succeed when other drugs have failed.
DRONEDARONE’S PHARMACOLOGY
Dronedarone, like amiodarone, blocks multiple sodium and potassium ion channels. It also exerts an antiadrenergic effect by noncompetitive binding to beta-adrenergic receptors as well as by inhibiting an agonist-induced increase in adenylate cyclase activity.8 Compared with amiodarone, dronedarone is a more potent blocker of peak sodium current.
Dronedarone is largely metabolized by the hepatic enzyme cytochrome P450 3A4 isoform (CYP3A4). Only 6% of dronedarone is excreted renally; however, no trial has yet assessed dronedarone’s safety in patients with marked kidney dysfunction.89
Dronedarone’s steady-state terminal elimination half-life is approximately 30 hours. When taken twice a day, it achieves steady-state concentrations in 5 to 7 days.
Dronedarone is available only for oral administration at 400 mg twice daily. Dose adjustment or titration is not recommended.
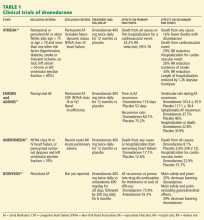
CLINICAL TRIALS OF DRONEDARONE
Dronedarone vs placebo
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death From any Cause in Patients With Atrial Fibrillation/Atrial Flutter)10 was a prospective, double-blind study to assess morbidity and death rates in 4,628 patients with atrial fibrillation or atrial flutter and at least one other cardiovascular risk factor.
EURIDIS and ADONIS. Two trials,11 EURIDIS (European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm) and ADONIS (American-Australian Trial With Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm), enrolled a total of more than 1,200 patients and showed that dronedarone 400 mg twice a day produced a significantly lower rate of recurrence of atrial fibrillation after electrical cardioversion compared with placebo.
Overall, treatment with dronedarone significantly reduced the risk of a first recurrence of atrial fibrillation by 22% (ADONIS) and 27.5% (EURIDIS) (Table 1).
ERATO (Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation),12 an additional phase III study, showed that dronedarone controlled the heart rate in patients with persistently accelerated ventricular rates despite concomitant standard therapy with a beta-blocker, digitalis, or a calcium-channel blocker. Dronedarone reduced the mean 24-hour heart rate by 11.7 beats per minute and the maximal exercise ventricular rate by 24.5 beats per minute at the 14th day.
ANDROMEDA (Anti-arrhythmic Trial With Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease)13 was a study not of patients with atrial fibrillation but rather of patients with symptomatic congestive heart failure, a left ventricular ejection fraction of 35% or less, and recent hospitalization with new or worsening heart failure. The study was terminated early because of a higher rate of death with dronedarone13 (Table 1).
Dronedarone vs amiodarone
DIONYSOS (Efficacy and Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation)14 was a randomized double-blind trial. It evaluated the efficacy and safety of dronedarone (400 mg twice daily) or amiodarone (600 mg daily for 28 days, then 200 mg daily thereafter) for at least 6 months for the maintenance of sinus rhythm in patients with atrial fibrillation. It enrolled 504 patients with persistent atrial fibrillation; patients had not previously taken amiodarone. Dronedarone was less effective than amiodarone in maintaining sinus rhythm: the rate of recurrent atrial fibrillation was 63% with dronedarone and 42% with amiodarone. But dronedarone was associated with fewer adverse effects and less need for premature discontinuation of drug treatment at a mean follow-up of 7 months (Table 1).
WHERE DOES DRONEDARONE FIT IN ATRIAL FIBRILLATION MANAGEMENT?
Dronedarone is indicated in persistent or paroxysmal atrial fibrillation, based on the observed reduction of the rate of hospitalization. It is indicated for the maintenance of sinus rhythm and may be used in patients with persistent or paroxysmal atrial fibrillation and flutter who are in sinus rhythm or will be undergoing cardioversion soon after starting the drug. Dronedarone has no role in the acute management of atrial fibrillation, such as in cardioversion to sinus rhythm in the emergency department.
We do not have substantial evidence of the efficacy of dronedarone in patients with resistant atrial fibrillation, in whom multiple antiarrhythmics have failed to maintain sinus rhythm, and no published trial has used the inclusion criterion of treatment failure with other antiarrhythmic drugs.
The role of dronedarone in heart failure with preserved systolic function is unclear. Patients taking dronedarone are twice as likely as those taking amiodarone to have a recurrence of atrial fibrillation.
The main advantage of dronedarone is its lower adverse effect profile. However, this statement is based on only a few years of observation. If the patient has developed adverse effects with amiodarone, or if the clinician is concerned about the risk of serious adverse effects, dronedarone presents an alternative for those patients without heart failure or significant left ventricular dysfunction. One such group may be younger patients, because of concerns about the cumulative effects of amiodarone taken over a lifetime.
Dronedarone may represent an acceptable alternative to many of the current antiarrhythmic drugs. Based on the results of the Cardiac Arrhythmia Suppression Trial (CAST),15 class IC antiarrhythmics such as flecainide (Tambocor) are generally avoided in patients with prior myocardial infarction or with known or even suspected coronary artery disease. Similarly, sotalol (Betapace) is generally avoided in patients with marked left ventricular hypertrophy because of adverse effects.16 Dofetilide (Tikosyn) and often sotalol require hospitalization with telemetric monitoring for QTc prolongation and the risk of proarrhythmia with torsades de pointes. Dronedarone, however, generally can be safely started in the outpatient setting.
As when considering prescribing any antiarrhythmic, the clinician must assess the patient’s thromboembolic risk, since this risk persists with a rhythm control strategy.
There is substantial evidence from the ATHENA trial,10 in which 30% of the patients had coronary artery disease, that dronedarone is safe and effective in patients with coronary artery disease. Its use in patients who have undergone coronary artery bypass surgery remains to be defined.