Aortic dissection: Prompt diagnosis and emergency treatment are critical
ABSTRACTDiagnosing aortic dissection requires a high index of suspicion, as it may mimic other more common conditions that cause chest pain. Prompt diagnosis is key, as it requires emergency evaluation and treatment for optimal chances of survival. This paper reviews key clinical features as well as laboratory and imaging tests.
KEY POINTS
- Aortic surgery is the treatment of choice for dissection of the ascending aorta, whereas dissection of the descending aorta is initially managed medically.
- Look for an underlying genetic predisposition to aortic disease and, in many instances, screen first-degree relatives for aortic disease.
- Long-term management requires serial imaging of the aorta, blood pressure control, and, for many, future aortic procedures.
- Measuring the D-dimer levels may help in decision-making for appropriate imaging in patients presenting with chest pain, as an elevated level raises the suspicion of dissection. However, more study of this and other biomarkers is needed.
- Advances in molecular genetics and the biology of the aortic wall promise to improve the diagnosis and prognosis of aortic disease.
DEFINITIVE IMAGING STUDIES: CT, MRI, TEE
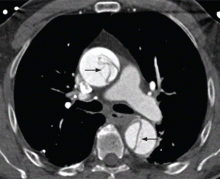
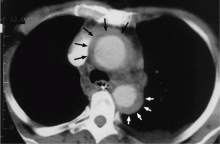
Contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), and transesophageal echocardiography (TEE) all have very high sensitivity and specificity for the diagnosis of aortic dissection.2,3 The choice of imaging study often depends on the availability of these studies, with CT and TEE being the most commonly performed initial studies.
MRI is outstanding for detecting and following aortic dissection, but it is usually not the initial study performed because of the time required for image acquisition and because it is generally not available on an emergency basis.
While transthoracic echocardiography (TTE) can detect aortic dissection, its sensitivity is much lower than that of other imaging tests.2,3 Therefore, negative findings on TTE do not exclude aortic dissection.
MANAGEMENT OF AORTIC DISSECTION
When acute aortic dissection is diagnosed, multidisciplinary evaluation and treatment are necessary. Time is of the essence, as the death rate in acute dissection may be as high as 1% per hour during the first 24 hours.1–3 All patients with acute aortic dissection, whether type A or type B, should be transferred to a tertiary care center with a staff experienced in managing aortic dissection and its complications.3 Emergency surgery is recommended for type A aortic dissection, whereas type B dissection is generally treated medically unless complications occur.2,3
The cornerstone of drug therapy is the prompt reduction in blood pressure with a beta-blocker to reduce shear stresses on the aorta. Intravenous agents such as esmolol (Brevibloc) or labetalol (Normodyne) are usually chosen. Sodium nitroprusside may be added to beta-blocker therapy for rapid blood pressure control in appropriate patients. The patient may require multiple antihypertensive medications. If hypertension is refractory, one must consider renal artery hypertension due to the dissection causing renal malperfusion.2 Acute pain may also worsen hypertension, and appropriate analgesia should be used.
Definitive therapy in acute dissection
Patients with acute type A dissection require emergency surgery,2,3 as they are at risk for life-threatening complications including cardiac tamponade from hemopericardium, aortic rupture, stroke, visceral ischemia, and heart failure due to severe aortic regurgitation. When aortic regurgitation complicates acute type A dissection, some patients are adequately treated by resuspension of the aortic valve leaflets, while others require valve-sparing root replacement or prosthetic aortic valve replacement.
Surgical therapy is associated with a survival benefit compared with medical therapy in acute type A dissection.1 The 14-day mortality rate for acute type A dissection treated surgically is about 25%.1 Patients with high-risk features such as heart failure, shock, tamponade, and mesenteric ischemia have a worse prognosis compared with those without these features.2,12,13
Acute type B aortic dissection carries a lower rate of death than type A dissection.1–3 In the IRAD cohort, the early mortality rate in those with type B dissection treated medically was about 10%.1 However, when complications such as malperfusion, shock, or requirement for surgery occur in type B dissection, the mortality rate is much higher,2,14 with rates of 25% to 50% reported.2
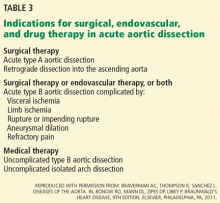
Thus, initial medical therapy is the preferred approach to acute type B dissection, and surgery or endovascular therapy is reserved for patients with acute complications.2,3 Typical indications for surgery or endovascular therapy in type B dissection include visceral or limb ischemia, aortic rupture, refractory pain, and aneurysmal dilation (Table 3).2
Endovascular therapy in aortic dissection
The high mortality rate with open surgery in acute type B dissection has spurred tremendous interest in endovascular treatments for complications involving the descending aorta and branch vessels.2
Fenestration of the aorta and stenting of branch vessels were the earliest techniques used in complicated type B dissection. By fenestrating (ie, opening) the intimal flap, blood can flow from the false lumen into the true lumen, decompressing the distended false lumen.
Endovascular stenting is used for acute aortic rupture, for malperfusion syndromes, and for rapidly enlarging false lumens. Endovascular grafts may cover the area of a primary intimal tear and thus eliminate the flow into the false channel and promote false-lumen thrombosis. Many patients with complicated type B dissection are treated with a hybrid approach, in which one segment of the aorta, such as the aortic arch, is treated surgically, while the descending aorta receives an endovascular graft.2
Patients with a type B dissection treated medically are at risk for late complications, including aneurysmal enlargement and subsequent aortic rupture. The Investigation of Stent Grafts in Aortic Dissection (INSTEAD) trial included 140 patients with uncomplicated type B dissection and compared drug therapy with endovascular stent grafting.15 After 2 years of follow-up, there was no difference in the rate of death between the two treatment groups. Patients receiving endovascular grafts had a higher rate of false-lumen thrombosis.
More studies are under way to examine the role of endovascular therapy in uncomplicated type B dissection.