Proton pump inhibitor side effects and drug interactions: Much ado about nothing?
ABSTRACTProton pump inhibitors (PPIs) are widely prescribed for acid-peptic disease. In general, the safety of this class of drugs has been excellent. However, in the past several years, epidemiologic studies have indicated possible risks that are biologically plausible.
KEY POINTS
- The US Food and Drug Administration has issued alerts that PPIs may increase the rate of osteoporosis-related fractures and may decrease the effectiveness of clopidogrel (Plavix) for preventing serious cardiovascular events.
- Other concerns include increased rates of pneumonia, Clostridium difficile infection, and other infections.
- A prudent approach to managing these concerns in day-to-day practice is required: PPIs, like any other drugs, should be prescribed only if indicated.
DO PPIs CAUSE OSTEOPOROSIS, FRACTURES?
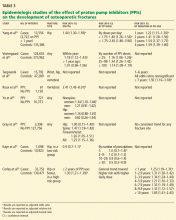
In a widely publicized paper published in 2006, Yang and colleagues25 reported the results of a large nested case-control study in the United Kingdom. The risk of hip fracture was significantly greater in patients who had been using PPIs for at least 1 year than in those who had not. The risk appeared to increase with longer use and higher doses of PPIs.
A similar risk of hip fracture was seen in a larger Danish case-control study published the same year.26 This study also found an increased odds ratio for PPI use in patients with spine fractures as well as in patients with any type of fracture. Interestingly, this study found a lower risk of fracture in patients using a histamine-2-receptor antagonist instead of a PPI.
Targownik et al27 found that the risk of hip fracture was not significantly higher until after 5 years of PPI exposure, with an even stronger risk after 7 years.
However, the data on both association and causal relationship are not uniform.
The Women’s Health Initiative,30 with more than 1 million person-years of followup, found no association between PPI use and hip fracture, but a modest association between PPI use and spine, arm, and wrist fractures, as well as total fractures.
A study in the United Kingdom found that patients without any major risk factors for hip fracture (defined by a risk ratio > 2) accounted for only 25% of cases but 53% of controls. When only these two average-risk groups were compared, the risk of hip fracture was similar in cases and controls.31
Corley et al32 also found that the risk of fracture associated with PPI use was only significant in the presence of another risk factor. These findings suggest that residual confounding may be to blame, at least in part, for the estimates of increased risk in the prior studies.
Another way to interpret these data is that PPIs increase the risk in patients at high risk to begin with, but not in those at average risk. This is an example of interaction (or effect modification) in which the risk is unequally distributed across groups with different characteristics.
A recently published study refutes the theory that impaired calcium absorption is responsible for the increase in fractures.33 In this study, investigators queried the Manitoba Bone Mineral Density Database to determine the relationship between antisecretory therapy with PPIs and osteoporosis or loss of bone mineral density—and they found none. This study may support the theory that residual confounding is the reason for the finding of an increased risk, but it also leaves open the possibility that PPIs induce other changes in bone microstructure that could increase the risk of fracture.
FDA labeling: Possible risk of fracture with PPIs
Based on the data so far, it appears possible that there is a small, albeit statistically significant, association between PPI use and fracture risk. The association is indeed biologically plausible, but it remains to be seen if this association is clinically significant, as the risk is relatively low. Even though the studies had methodologic limitations, on May 25, 2010, the FDA announced a change in the required labeling information for PPIs to indicate a possible risk of fracture with these drugs.34
Reassess the need for chronic PPI therapy
Although patients may worry that they will develop osteoporosis and fractures if they take PPIs, the data do not support a strong risk. Nevertheless, when faced with a patient on chronic PPI therapy, especially with a high dose, providers should use the opportunity to reassess the indication for the PPI to decide if chronic therapy is required, in a matter similar to the algorithm provided for PPI-clopidogrel cotherapy (FIGURE 2). Providers should educate patients about the data, and limit new and recurring PPI prescriptions to patients who require a PPI for appropriate indications, at the lowest dose, and for the shortest time possible.
DO PPIs INCREASE THE RISK OF PNEUMONIA?
Several recent studies have also raised concern about an association between PPI use and pneumonia.
Normally, the stomach remains free of bacteria (except for Helicobacter pylori) because its acidic milieu destroys nearly all bacteria swallowed. If the stomach becomes less acidic, it loses this protective mechanism, and ingested organisms can survive and proliferate.35 In theory, when gastroesophageal reflux occurs, these bacteria could be carried up to the hypopharynx where microaspiration into the lower airways could lead to pneumonia, especially in patients with compromised oropharyngeal protective reflexes (eg, patients on mechanical ventilation).
This possible association came to the attention of the general medical community when a Dutch study,36 in which 5,551 cases of community-acquired pneumonia developed in 364,683 people, found that the incidence of pneumonia was about 4.5 times higher in people exposed to acid-suppressive drugs (both PPIs and histamine-2-receptor antagonists) than in unexposed individuals. Patients who developed pneumonia also had higher odds of significant comorbid conditions, including heart failure and chronic obstructive pulmonary disease. The authors calculated that about one case of pneumonia per 226 patients treated with a PPI would be attributable to the PPI. A major limitation of this study, however, was that only 18% of the patients diagnosed with pneumonia actually had radiologic or microbiologic confirmation of pneumonia.
Other studies later examined the relationship between PPIs and community-acquired pneumonia,37–41 and most have revealed a modestly higher risk of community-acquired pneumonia in patients exposed to PPIs.
This risk was confirmed in a recent metaanalysis, which found a higher risk of community-acquired pneumonia with PPI use (odds ratio 1.36, 95% CI 1.12–1.65).42 However, the authors refrained from drawing definitive conclusions from these data because of significant heterogeneity between the studies. One study37 found that recent onset of use (within 7 days) had a much stronger association with community-acquired pneumonia than longer-term use, which is contradictory to a causal association, since longer-term use should lead to more cases of pneumonia.
Another study investigated the association between acid-suppressive drugs and hospital-acquired pneumonia in nonventilated patients.43 In a 4-year period, there were 63,878 admissions in 42,093 unique patients. Acid-suppressive drugs were prescribed in 32,922 admissions (52%); the drugs included PPIs in 83% of these. Hospital-acquired pneumonia occurred in 2,219 admissions (3.5%), with a higher incidence in patients exposed to acid-suppressive drugs than in the unexposed group (4.6% vs 2.0%). The adjusted odds ratio for pneumonia was 1.3 (95% CI 1.1–1.4) in the exposed group. Subgroup analysis revealed that the association remained significant for PPIs but not for histamine-2-receptor antagonists.
Adequate studies of mechanically ventilated patients in the current era of intravenous PPI use are lacking. Older studies in this group of patients may not be generalizable to current practice because of the reduction in gastric volume with intravenous PPIs that may offset the theoretical risk of aspiration.35
Although the data supporting the association are not exceedingly strong, the relationship is biologically plausible. If there is a risk, it seems to be greatest in the sickest patients, who can least afford to develop pneumonia. Therefore, prudent prescribing should be the rule for both inpatients and outpatients, especially in patients with comorbidities, in whom pneumonia could have serious consequences.