Interferon-gamma-release assays: Better than tuberculin skin testing?
ABSTRACTAlthough the tuberculin skin test has long been the standard for detecting latent tuberculosis infection, it has many limitations. Interferon-gamma-release assays are gaining acceptance as an alternative. In this paper we present cases to illustrate how these new tests can be used and how to interpret the results.
KEY POINTS
- Prior vaccination with bacille Calmette-Guérin can cause the results of skin testing to be falsely positive, but it does not affect interferon-gamma-release assays.
- In 2005, the US Centers for Disease Control and Prevention recommended that interferon-gamma-release assays be used in all situations in which skin testing is currently used. Updated guidelines were published on June 25, 2010.
- Successful implementation of interferon-gamma-release assay testing requires education of everyone involved—phlebotomists, laboratory personnel, occupational health workers, and clinicians.
Tuberculin skin testing, long the standard method for detecting latent tuberculosis,1,2 has well-known limitations. Prior vaccination with bacille Calmette-Guérin (BCG) or exposure to other nontuberculous mycobacterial species can cause false-positive results.1,3 Errors can occur in the intradermal placement and the reading of the test. The patient must return in 48 to 72 hours for an accurate reading of the test. False-negative results can occur in severe illness or immunosuppression. And a “booster response” can occur, in which immunologic memory of an earlier skin test can provoke a false-positive response.1,3–5
Interferon-gamma-release assays are an alternative. The QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) was approved by the US Food and Drug Administration in 2001. Subsequently, two other tests were approved and are now commercially available:
- QuantiFERON-TB Gold In-Tube (QFTGIT) (Cellestis)
- T-SPOT.TB (Oxford Immunotec, Marlborough, MA).
We discuss how these tests work, focusing mainly on the QFT-GIT, and we present several cases to illustrate how they are used in preemployment screening and in sequential-testing surveillance programs for health care workers, and potential challenges in interpreting the results.
HOW THE NEW ASSAYS COMPARE WITH TUBERCULIN SKIN TESTING
Unlike tuberculin skin testing, interferongamma-release assays are blood tests.1
Either whole blood (in the QuantiFERON tests) or peripheral blood mononuclear cells (in the T-SPOT.TB test) are incubated with various tuberculosis-specific antigens. In response to the antigens, effector T cells produce interferon-gamma, which is measured quantitatively and qualitatively by either enzyme-linked immunosorbent assay (in the QuantiFERON tests) or enzymelinked immunospot assay (in the T-SPOT. TB test).1,6,7
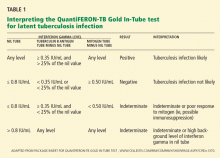
The kit for the QFT-GIT test,6 which we use, contains three heparinized tubes for blood collection:
- A control (“nil”) tube, which contains no antigens. The purpose of this tube is to determine the patient’s “baseline” level of interferon gamma.
- A tube containing tuberculin antigens (ESAT-6, CFP-10, and TB7.7). When blood from patients who were previously exposed to Mycobacterium tuberculosis is incubated in this tube, the T cells recognizing the tuberculin antigen produce significant amounts of interferon gamma, and levels go up above that in the control tube. The level should not increase in patients not exposed to this organism.
- A tube containing mitogen, a nonspecific stimulant of interferon gamma production. This tube represents a “positive” control.
These tests appear to be unaffected by previous BCG vaccination, unlike tuberculin skin testing. A meta-analysis in 2008 reported a pooled specificity of 98% for the QuantiFERON tests: 99% in patients not vaccinated with BCG, and 96% in BCG-vaccinated patients. 8 The analysis also concluded that the T-SPOT.TB test appears to be more sensitive for latent tuberculosis than the QuantiFERON tests or tuberculin skin testing.8
HOW SHOULD THESE NEW TESTS BE USED?
In 2005 and in 2010, the US Centers for Disease Control and Prevention (CDC) recommended that interferon-gamma-release assays be used in all situations in which the skin test is currently used, “including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control,”9 such as for health care workers. The UK National Institute for Clinical Excellence has taken a more conservative approach, suggesting that they be used only as adjuvants to tuberculin skin testing.10
In 2007, Cleveland Clinic began using the QFT-GIT test instead of the skin test for preemployment screening of health care workers for latent tuberculosis, and these workers will continue to be screened once a year with this test. Employees hired before 2007 are still being screened every year by skin testing. The number of health care workers with latent tuberculosis infection accepting isoniazid treatment for it increased when assay testing was implemented along with a process for counseling and providing treatment.11
Converting from tuberculin skin testing to interferon-gamma-release assays poses challenges. Phlebotomists need to be trained in how to collect and process the blood. Specimens must be received in the laboratory within 16 hours of collection, which may require courier service.12 Other considerations include availability of a laboratory that can process the assays.1 Also, these tests cost substantially more than the tuberculin skin test. However, one recent cost-benefit analysis13 found that in screening programs for healthcare workers, using interferon gamma release assays was clinically superior and more cost-effective than skin testing.
In the following sections, we present cases that illustrate how these new tests are used in the diagnosis of latent tuberculosis, and potential challenges in interpretation of results. We will not discuss their use for diagnosing active tuberculosis.